Your Location:Home >Products >Functional intermediates >604-53-5
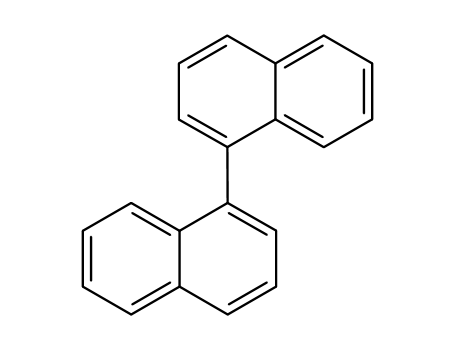

Product Details
Chemical Properties
Brown powder
Purification Methods
Purify 1,1’-binaphthyl through a silica gel column with Me2CO/*C6H6 [or Al2O3 with 10% *C6H6/pet ether (b 30-60o)] and recrystallise it from EtOH, pentane, or slow evaporation of *C6H6, Me2CO or Et2O solutions. Half life ~10hours at 25o in various solvents. [Wilson & Pincock J Am Chem Soc 97 1474 1975, Akimoto & Yamada Tetrahedron 27 5999 1971, Beilstein 5 I 358, 5 II 642, 5 III 2465, 5 IV 2634.]
The rates of atropisomerization of (S)-1,1'-binaphtyl (BN) are compared in six nematic phases and in the solid phase of p-methoxybenzylidene-p-n-butylaniline.The influence of each phase on the activation parameters for atropisomerization are correlated with the solvent molecular structures.The results in the nematic and solid phases are interpreted in terms of degree to which the solvent matrices flatten the angle between the naphtyl rings of BN.
The Suzuki arylation of enantiopure 2,2′-diiodo-1,1′- binaphthalene affords the 2,2′-diarylated products in considerable yields (up to 52%), however, significantly racemized. The reversed-polarity approach, using novel enantiopure 1,1′-binaphthalene-2,2′-diyldiboronic acid, prepared either by resolution or by stereoconservative boronation, allowed, after optimization of coupling conditions, to obtain the model 2,2′-ditolylated product in good yield (56%) as well, but in addition, without impairing of enantiomeric purity (i.e. stereo-conservatively). The developed synthetic approach was found to be an expedient method for the synthesis of enantiopure 2,2′-diaryl-1,1′-binaphthalenes, especially for those with electron-neutral and electron-deficient poor aryl groups. Observing that the diboronic acid decomposes by hydrodeboronation under the reaction conditions, 2-aryl-1,1′-binaphthalenes were isolated as the main products from the reaction with less reactive electron-rich aryl iodides.
-
The cathodic reduction of aryl iodides ArIs at carbon electrodes was achieved in cyclic carbonates (e.g., propylene carbonate PC). The novelty of the present method simply lies in the ability of title compounds to create aromatic carbanions that may act as nucleophiles towards carbonates. Consequently, several aromatic esters ArC(O)OR are generated as side products that play the role of redox mediators for indirectly cleaving C-X bond at solid inert electrodes. This procedure surprisingly allows one to obtain, through this self-induced redox catalysis, Ar-Ar dimers sometimes in high yield. Meanwhile, the triggering of such processes could be boosted by doping inert electrodes by electro-deposition of metals such as palladium, nickel, or silver. Extremely thin layers of metals are well shown to be very efficient (even at average thickness 0.1 nm) to induce catalytic steps presenting quite large potential shifts compared to bare carbon electrodes when employed in inert solvents. The probable concomitance of electrocatalytic and redox catalytic processes is discussed and argued.
Suzuki-Miyaura reactions were performed over Pd loaded on (ultra-stable Y) USY zeolites prepared by steam treatment of NH4-Y. We found that the catalytic activity of Pd increased significantly with steam treatment of NH 4-Y when 6% H2 was applied before and during the reactions. For instance, a TON of 13,000,000 was obtained in the reaction between bromobenzene and phenylboronic acid in 1.5 h. Pd K-edge and Pd L 3-edge X-ray absorption fine structure analyses revealed the formation of atomic Pd with a cationic character. The catalytic activity of Pd/USY prepared under different steam-treatment conditions was in good correlation with the strong Bronsted acid sites induced by the extra-framework Al. Based on the catalytic performance data, the structure of Pd, and acidic analysis of the support, atomic Pd anchored to the strong Bronsted acid sites of the USY zeolite was proposed to be the active species.
The 4-aryl substituted pyrazolate ligands, L1(Ph)H and L1(Naph)H, were synthesized by SuzukiMiyaura cross-coupling and combined with metal sources to give cyclic trinuclear structures [AgL1(Ph)]3, [AuL1(Ph)] 3, [AgL1(Naph)] 3, and [AuL1(Naph)]3. The aryl group determined the crystal packing of these cyclic trinuclear complexes with the phenyl systems exhibiting stair type solid state structures and the naphthyl complexes exhibiting irregular structures with spaces occupied with some solvent molecules. These differences in solid-state structures were accompanied by differences in MN stretching frequencies and temperature dependent photoluminescence.
Viedma ripening is a process that combines abrasive grinding of a slurry of crystals with solution-phase racemization, resulting in solid-phase deracemization. One of the major disadvantages of Viedma ripening is that the desired compound needs to crystallize as a racemic conglomerate, accounting for only 5–10 % of all chiral molecules. Herein, we show that use of a chiral additive causes deracemization under conditions, in which the compound normally crystallizes as a racemic compound. Although this concerns a single example, it is envisioned that through this new approach the scope of Viedma ripening can be significantly expanded.
-
Palladium catalysed, aryl boronic acid homocoupling, is explored as a fluorescence sensing regime for saccharides. The catalytic formation rate of fluorescent bi-aryls, under control of a palladium catalyst, is modulated by the presence of saccharides. The nature of the aryl group, rate of biaryl formation and limits of detection are investigated.
The racemization of 1,1'-binaphthyl in ethanol is subject to heterogeneous catalysis by platinum (prepared by reduction of platinum oxide with hydrogen).The rate of catalyzed reaction is first order in binaphthyl but independent of platinum concentration over a limited range.First-order rate constants at 25 deg C decrease with increased binaphthyl concentration and are up to 12-fold that for the uncatalyzed racemization.Catalysis by platinum is stopped momentarily by the injection of air and is diminished by injection of cyclohexene or cyclohexane into the solution.Racemization therefore occurs on active sites which are also capable of reducing oxygen or cyclohexene.It is suggested that, for racemization of binaphthyl, these sites are acting as electron donors rather than hydrogen atom donors.
Abstract: A combination of Pd and Ni complexes activated aryl bromides for the thermal Mizoroki-Heck reaction and Suzuki coupling giving high yields in short reaction times. A thermal redox mechanism probably occurs whereby Ni complex transfers electron and reduces the Pd (II) to Pd (0) which then takes the reactants through the standard protocol of oxidative-addition, migratory insertion and reductive elimination, typical for the Mizoroki-Heck reaction and the Suzuki coupling. Graphic Abstract: [Figure not available: see fulltext.]
A nickel-catalyzed amination to access diarylamines has been developed through C?CN bond activation of aryl nitriles with anilines. In this developed catalytic protocol, various aromatic and heteroaromatic nitriles could be utilized as the electrophiles to couple with substituted anilines. A diversity of diarylamines were obtained in 15–95% yields. (Figure presented.).
MeOTf-catalyzed formal [4 + 2] annulation of styrene oxides with alkynes to afford polysubstituted naphthalenes has been realized, which undergoes sequential electrophilic cyclization/ring expansion. A range of substrates were tolerated in the formation of naphthalene derivatives with high regioselectivity in satisfactory yields. The reaction could also be carried out on gram scale.
Pairing lithium and manganese(II) to form lithium manganate [Li2Mn(CH2SiMe3)4] enables the efficient direct Mn–I exchange of aryliodides, affording transient (aryl)lithium manganate intermediates which in turn undergo spontaneous C?C homocoupling at room temperature to furnish symmetrical (bis)aryls in good yields under mild reaction conditions. The combination of EPR with X-ray crystallographic studies has revealed the mixed Li/Mn constitution of the organometallic intermediates involved in these reactions, including the homocoupling step which had previously been thought to occur via a single-metal Mn aryl species. These studies show Li and Mn working together in a synergistic manner to facilitate both the Mn–I exchange and the C?C bond-forming steps. Both steps are carefully synchronized, with the concomitant generation of the alkyliodide ICH2SiMe3 during the Mn–I exchange being essential to the aryl homocoupling process, wherein it serves as an in situ generated oxidant.
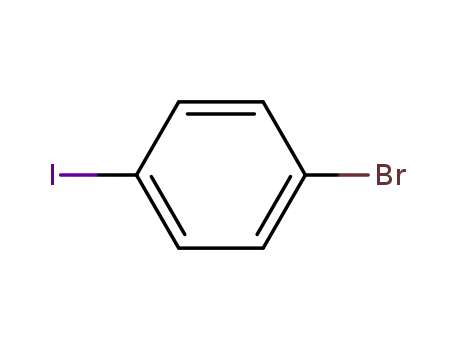
1,4-bromoiodobenzene

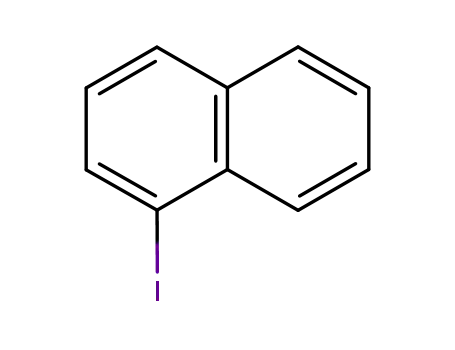
1-Iodonaphthalene

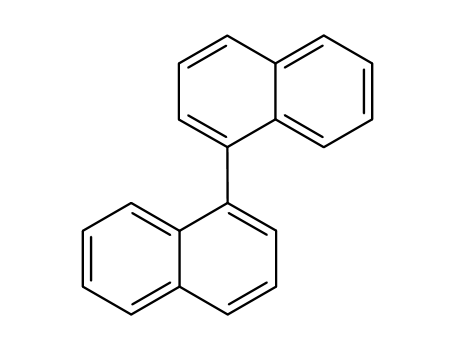
1,1'-bisnaphthalene

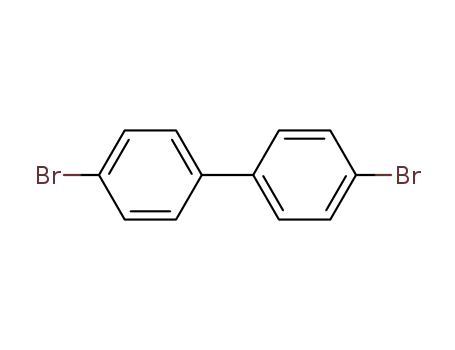
4-(4-bromophenyl)bromobenzene


1-(4-bromo-phenyl)-naphthalene
| Conditions | Yield |
|---|---|
|
With
palladium diacetate; potassium carbonate;
In
butanone;
at 120 ℃;
for 12h;
Inert atmosphere;
|
55% 11 %Chromat. 16 %Chromat. |
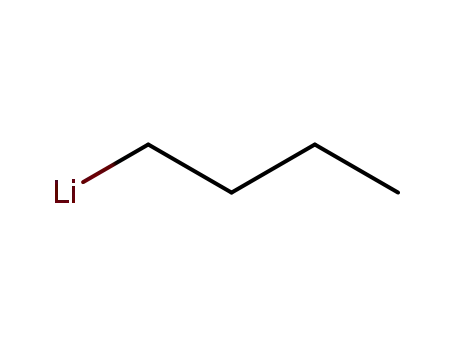
n-butyllithium

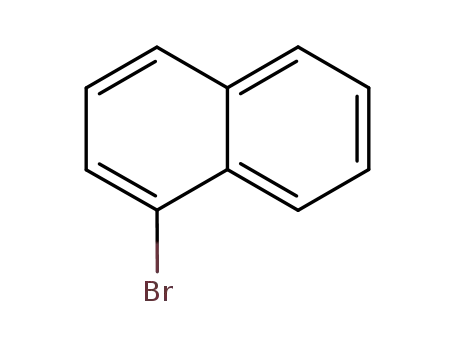
1-Bromonaphthalene

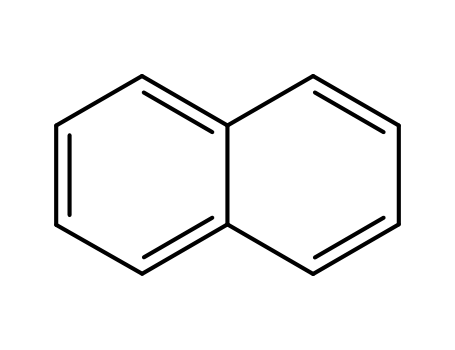
naphthalene


1,1'-bisnaphthalene

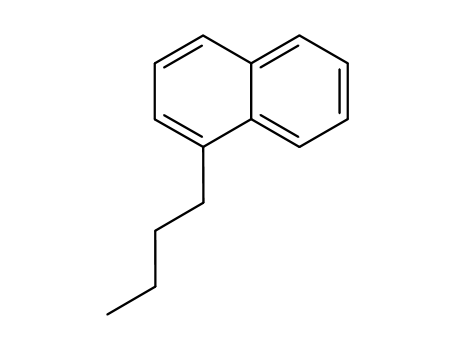
1-butylnaphthalene
| Conditions | Yield |
|---|---|
|
With
di-μ-iodobis(tri-t-butylphosphino)dipalladium(l);
In
tetrahydrofuran; toluene;
at 20 ℃;
for 0.0833333h;
chemoselective reaction;
|
62 %Chromat. 24 %Chromat. 14 %Chromat. |

naphthalene
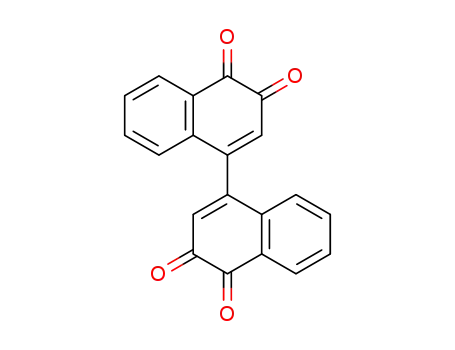
[1,1'-binaphthalene]-3,3',4,4'-tetraone

1-Bromonaphthalene

1-Iodonaphthalene
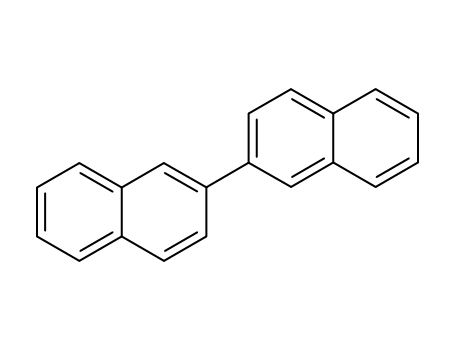
2,2'-binaphthalene
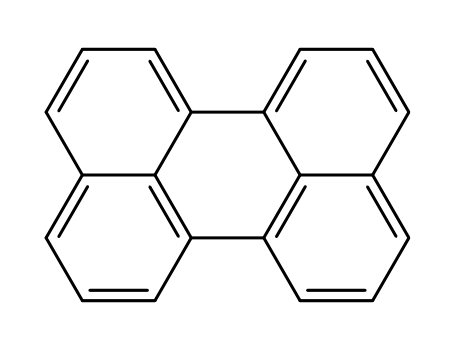
PERYLENE
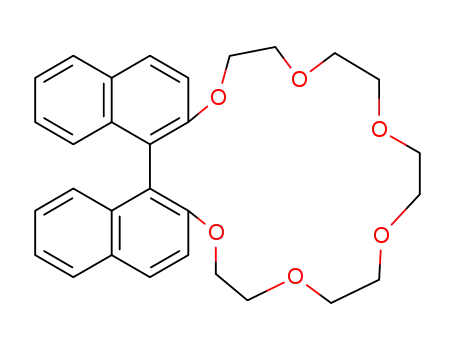
2,2'-binaphthylidyl-20-crown-6

naphthalene
CAS:13885-09-1
CAS:400607-04-7
Molecular Formula:C24H15Br
Molecular Weight:383.3