Your Location:Home >Products >Functional intermediates >35225-79-7
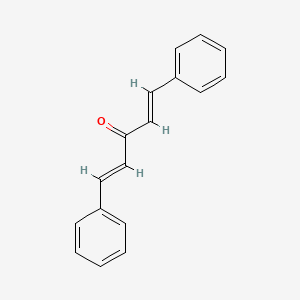

Product Details
|
Chemical Properties |
trans,trans-Dibenzylideneacetone is a yellow crystalline powder, melting point 110-111℃; cis-trans is a light yellow needle crystal, melting point 60℃; cis-cis is a yellow oily liquid, boiling point 130℃ (2.7Pa). Soluble in ethanol, acetone, chloroform, insoluble in water. |
|
Description |
Dibenzylidene acetone also known as dibenzalacetone and abbreviated as dba, is an organic compound. It appears as a bright-yellow solid that is insoluble in water but soluble in ethanol. First synthesized in 1881 by chemists Rainer Ludwig Claisen and Charles-Claude-Alexandre Claparède, dibenzylideneacetone is utilized as a component in sunscreens and as a ligand in organometallic chemistry. |
| Uses |
Dibenzylidene acetone is primarily used as a component in sunscreens and as a ligand in organometallic chemistry. Notably, it is part of the catalyst tris(dibenzylideneacetone)dipalladium(0), where it acts as a labile ligand easily displaced by stronger ligands such as triphenylphosphine, facilitating palladium(0) chemistry. Additionally, dba is involved in various chemical reactions, including copper-catalyzed N-arylation of imidazoles, Nazarov-like cyclization, transfer hydrogenation, Lewis acid mediated condensation, Hetero-Diels-Alder reactions, asymmetric 1,4-addition reactions, and Michael addition reactions. |
InChI:InChI=1/C17H14O/c18-17(13-11-15-7-3-1-4-8-15)14-12-16-9-5-2-6-10-16/h1-14H/b13-11+,14-12u
In this work, we have evaluated the full 2PA spectra using femtosecond pulses of a chalcone derivative and a series of dibenzylideneacetone and thiosemicarbazone derivatives in a dichloromethane medium.
A particular subset of dibenzylideneacetone (DBA) compounds exhibited potent in vitro antitrypanosomal activity with sub-micromolar EC50 values. A structure–activity relationship study including 26 DBA analogs was initiated, and several compounds exhibited EC50 values as low as 200 nM.
benzaldehyde
isopropyl alcohol
α-chlorobenzyl methyl ether
acetone
1t,5-diphenyl-5-piperidino-pent-1-en-3-one
1,5-Diphenyl-3-oxo-5-morpholino-pent-1-en
1,5-di-morpholin-4-yl-1,5-diphenyl-pentan-3-one
4-methyl-4-nitro-3,5-diphenyl-cyclohexanone
CAS:120-74-1
Molecular Formula:C<sub>8</sub>H<sub>10</sub>O<sub>2</sub>
Molecular Weight:138.16
CAS:538-58-9
CAS:604-53-5