Your Location:Home >Products >Organic phosphines >Phenyl phosphines >261733-18-0
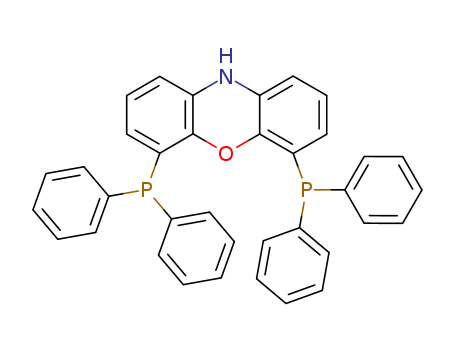

Product Details
Reactions
A large bite-angle chelating bisphosphine that provides high levels of linear-to-branched selectivity in the hydroformylation of alkenes. A Deprotonatable Ligand for Room-Temperature Palladium-Catalyzed Cross-Couplings of Aryl Chlorides.
Uses
4,6-Bis(Diphenylphosphino)phenoxazine is used as a palladium catalyst in reactions involving the synthesis of triarylmethanes.
Uses
4,6-Bis(diphenylphosphino)phenoxazine used as an intermediate in the manufacture of pharmaceuticals, agrochemicals, dyestuffs, fine chemicals, medical and material synthesis.
Uses
N-XantPhos is a deprotonatable chelating aryldiphosphine ligand that can be used in:The preparation of Pd-NiXantphos catalyst system for the room temperature cross-coupling reactions of unactivated aryl chlorides.The synthesis of cinchonine iridium(III) cyclometalated complex that exhibits luminescence and good quantum efficiency.N-XantPhos and N-modified counterparts are also used in the preparation of rhodium based catalysts for hydroformylation reaction.
InChI:InChI=1/C36H27NOP2/c1-5-15-27(16-6-1)39(28-17-7-2-8-18-28)33-25-13-23-31-35(33)38-36-32(37-31)24-14-26-34(36)40(29-19-9-3-10-20-29)30-21-11-4-12-22-30/h1-26,37H
The invention discloses a synthetic method of a 4,6-di(diphenylphosphine)phenazine compound and belongs to the field of organic synthesis. In an anhydrous and oxygen-free atmosphere, bis(2-diphenylphosphinephenyl)ether is used as a raw material to react w
Although the past 15 years have witnessed the development of sterically bulky and electron-rich alkylphosphine ligands for palladium-catalyzed cross-couplings with aryl chlorides, examples of palladium catalysts based on either triarylphosphine or bidentate phosphine ligands for efficient room temperature cross-coupling reactions with unactivated aryl chlorides are rare. Herein we report a palladium catalyst based on NiXantphos, a deprotonatable chelating aryldiphosphine ligand, to oxidatively add unactivated aryl chlorides at room temperature. Surprisingly, comparison of an extensive array of ligands revealed that under the basic reaction conditions the resultant heterobimetallic Pd-NiXantphos catalyst system outperformed all the other mono- and bidentate ligands in a deprotonative cross-coupling process (DCCP) with aryl chlorides. The DCCP with aryl chlorides affords a variety of triarylmethane products, a class of compounds with various applications and interesting biological activity. Additionally, the DCCP exhibits remarkable chemoselectivity in the presence of aryl chloride substrates bearing heteroaryl groups and sensitive functional groups that are known to undergo 1,2-addition, aldol reaction, and O-, N-, enolate-α-, and C(sp2)-H arylations. The advantages and importance of the Pd-NiXantphos catalyst system outlined herein make it a valuable contribution for applications in Pd-catalyzed arylation reactions with aryl chlorides.
![bis[2-(diphenylphosphino)phenyl] ether](/upload/2023/2/428efe1f-202d-4d7f-8177-7bbcd9fa0b59.png)
bis[2-(diphenylphosphino)phenyl] ether

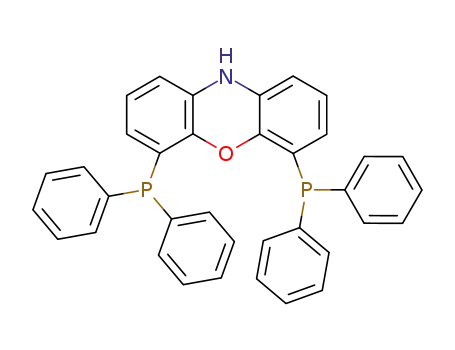
nixantphos
| Conditions | Yield |
|---|---|
|
bis[2-(diphenylphosphino)phenyl] ether;
With
tert.-butylhydroperoxide; sodium azide; copper(I) bromide;
In
water; acetonitrile;
at 30 ℃;
for 12h;
Inert atmosphere;
With
silver hexafluoroantimonate; dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer;
In
water; acetonitrile;
at 100 ℃;
for 18h;
Temperature;
Inert atmosphere;
|
92% |
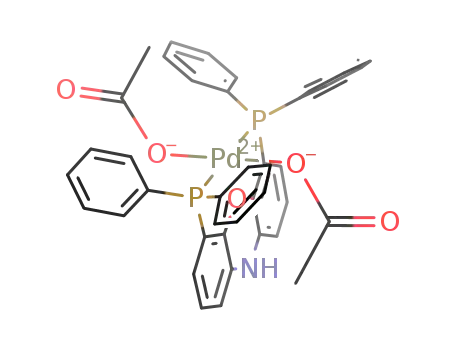
C40H33NO5P2Pd


nixantphos
| Conditions | Yield |
|---|---|
|
With
depe;
at 24 ℃;
for 0.666667h;
Inert atmosphere;
Glovebox;
|
88% |

C40H33NO5P2Pd
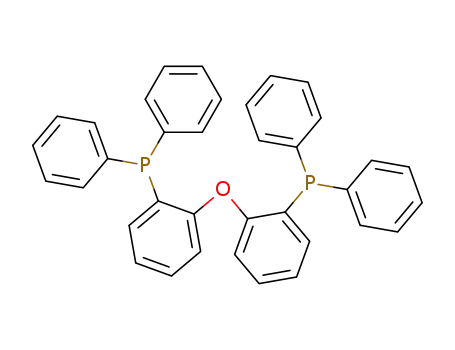
bis[2-(diphenylphosphino)phenyl] ether
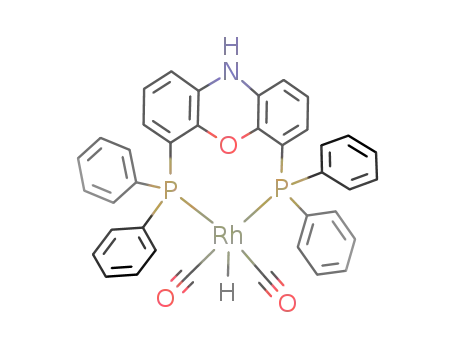
(nixantphos)Rh(CO)2H
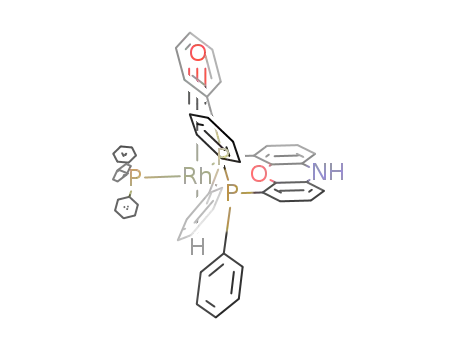
(nixantphos)Rh(CO)H(PPh3)
CAS:98327-87-8
Molecular Formula:C<sub>44</sub>H<sub>32</sub>P<sub>2</sub>
Molecular Weight:622.7
CAS:1770840-43-1
Molecular Formula:C6H5IN4
Molecular Weight:260.04
CAS:166330-10-5
Molecular Formula:C36H28OP2
Molecular Weight:538.6
CAS:161265-03-8
Molecular Formula:C39H32OP2
Molecular Weight:578.6