Your Location:Home >Products >OLED intermediates >Thiophenes >144012-09-9

Product Details
Chemical Properties
Light yellow liquid
In this work, we have designed and synthesized a new naphtho[1,2-b:5,6- b′]dithiophene-containing enlarged π-conjugated donor-acceptor (D-A) small molecule, NDT(TTz)2, for use in solution-processed organic photovoltaics. NDT(TTz)2, which contains a thiophene-bridged naphtho[1,2-b:5,6-b′]dithiophene as the central fused core and triphenylamine-flanked thiophene thiazolothiazole as a spacer, was synthesized via sequential Suzuki and Stille coupling reactions. The thermal, physiochemical, and electrochemical properties of NDT(TTz)2 have been evaluated by differential scanning calorimetry, thermogravimetry, UV-Vis spectroscopy, photoluminescence spectroscopy, X-ray diffraction, and cyclic voltammetry. As desired for photovoltaic applications, NDT(TTz)2 possesses good solubility, thermal stability, and a well-ordered, π-π stacked, crystallinity. The optical band gap and HOMO level of NDT(TTz) 2 were determined to be 2.0 eV and -5.23 eV, respectively. In addition to organic thin film transistor studies, application of NDT(TTz) 2 to preliminary photovoltaic devices has also been investigated by fabricating solution-processed bulk heterojunction solar cells together with PC71BM in a typical layered device structure, ITO/PEDOT:PSS/NDT(TTz) 2:PC71BM/LiF/Al. Without extensive optimization of the device, NDT(TTz)2 in these devices shows a maximum power conversion efficiency of 1.44% under AM 1.5 illumination at a 100 mW/cm2 intensity.
Two regioregular narrow bandgap conjugated polymers (PM1 and PM2) containing the repeat unit BDT-PT-CPDT-PT (BDT = benzodithiophene, PT = pyridyl[2,1,3]thiadiazole, CPDT = cyclopentadithiophene) and different solubilizing alkyl side chains were prepared with the goal of understanding how chemical structure impacts the performance of low Voc loss bulk heterojunction (BHJ) solar cells containing PC61BM as the acceptor semiconductor. Both polymers show nearly identical orbital energy levels, a face-on orientation relative to the surface normal, and can be processed to yield continuous fiber-like networks in the active layer. Due to the choice of repeat units within the backbone structure, PM1 and PM2 exhibit shorter π-π stacking distances, relative to the previously reported low Voc loss regioregular polymer PIPCP. Finally, PM1 achieves an average PCE of 6.2 ± 0.2% and PM2 achieves an average PCE of 7.2 ± 0.1%. Devices exhibit low Voc loss and high short circuit current Jsc, but, most significantly, display improved fill factors compared to previously reported PIPCP. A discussion is provided that seeks to identify structural features in conjugated polymers that lead to devices with low Voc loss and high external quantum efficiencies.
This paper reports the synthesis and the linear and non-linear absorption properties of a series of new tetrazine-based D-π-A-π-D and D-π-A type dyes. In these derivatives, a central tetrazine core was connected with one or two terminal triphenylamine moi
Conjugated systems built by connecting one electron-donor triphenylamine to an electron-withdrawing tetrazine have been prepared using various linkers. We describe here the synthesis, the electrochemical properties and some photophysical properties of the
A new bolaamphiphile comprising a 5,5′′′′′- diphenyl-sexithiophene core with glycerol groups at each end and four lateral decyl chains was synthesized, which self-assembles into a liquid crystalline phase representing a nanoscale honeycomb composed of qua

3-decylthiophene

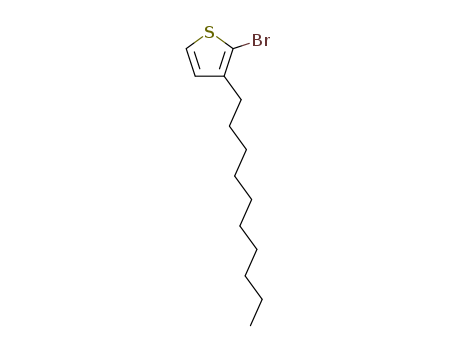
2-bromo-3-decylthiophene
| Conditions | Yield |
|---|---|
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 20 ℃;
for 15h;
|
91% |
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
at 20 ℃;
for 2h;
Inert atmosphere;
Darkness;
|
86% |
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform;
at 0 ℃;
for 17h;
Darkness;
|
69% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 0 ℃;
|
|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 12h;
|
|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
for 12h;
|
|
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 30 ℃;
for 15h;
|
|
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 25 ℃;
for 15h;
|
|
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform;
at 0 - 20 ℃;
Darkness;
|
1.9 g |
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
|
|
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 0 - 5 ℃;
for 15h;
|
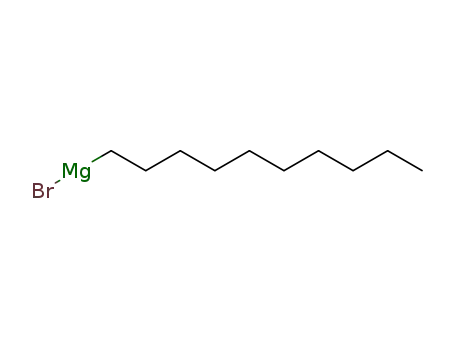
n-decyl magnesium bromide


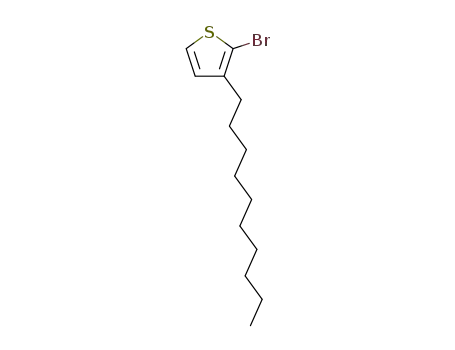
2-bromo-3-decylthiophene
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) / diethyl ether / 20 - 60 °C / Inert atmosphere
2: N-Bromosuccinimide / chloroform; acetic acid / 2 h / 20 °C / Inert atmosphere; Darkness
With
N-Bromosuccinimide; 1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
In
diethyl ether; chloroform; acetic acid;
|
|
|
Multi-step reaction with 2 steps
1: 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) / diethyl ether
2: N-Bromosuccinimide / N,N-dimethyl-formamide / 12 h / 20 °C
With
N-Bromosuccinimide; 1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
In
diethyl ether; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 2 steps
1: 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) / tetrahydrofuran / 15 h / Reflux
2: N-Bromosuccinimide / tetrahydrofuran / 15 h / 30 °C
With
N-Bromosuccinimide; 1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
In
tetrahydrofuran;
|
|
|
Multi-step reaction with 2 steps
1: 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) / tetrahydrofuran / 15 h / Reflux
2: N-Bromosuccinimide / tetrahydrofuran / 15 h / 25 °C
With
N-Bromosuccinimide; 1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
In
tetrahydrofuran;
1: |Kumada Cross-Coupling;
|
|
|
Multi-step reaction with 2 steps
1: 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) / tetrahydrofuran / 15 h / Reflux
2: N-Bromosuccinimide / tetrahydrofuran / 15 h / 0 - 5 °C
With
N-Bromosuccinimide; 1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
In
tetrahydrofuran;
1: |Kumada Cross-Coupling;
|

3-decylthiophene
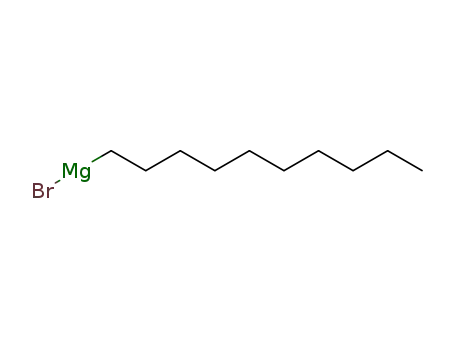
n-decyl magnesium bromide
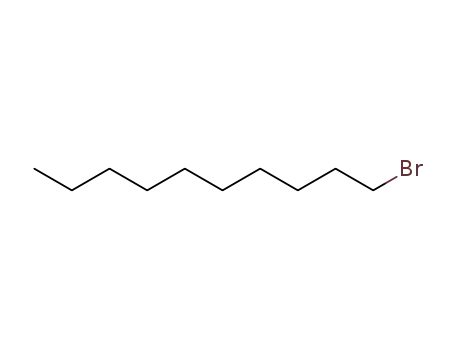
1-bromo dodecane
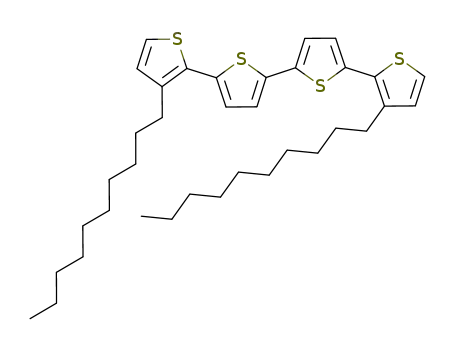
3,3'''-didecyl-2,2':5',2'':5'',2'''-quaterthiophene

3,3''-didecyl-2,2':5',2''-terthiophene
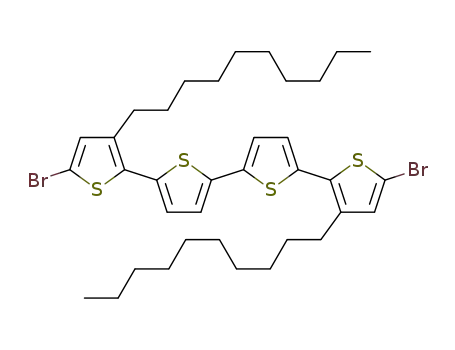
5,5'''-dibromo-3,3'''-didecyl-2,2':5',2'':5'',2'''-quaterthiophene
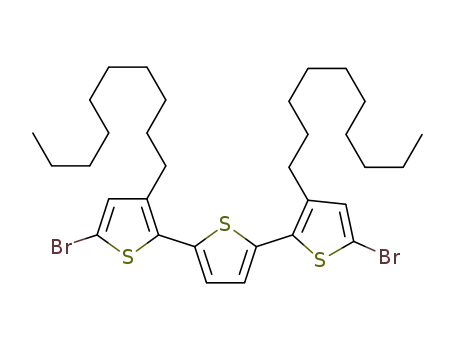
5,5''-dibromo-3,3''-didecyl-2,2':5',2''-terthiophene
CAS:864377-33-3
Molecular Formula:C18H14BNO2
Molecular Weight:287.1
CAS:65016-62-8
CAS:65016-55-9