Your Location:Home >Products >OLED intermediates >Thiophenes >65016-62-8


Product Details
Chemical Properties
clear colorless to light yellow liquid
Uses
3-Octylthiophene is used as a starting material in the synthesis of the following 2,5-dibromo-3-octylthiophene, poly(3-butylthiophene)-b-poly(3-octylthiophene), a diblock copoly(3-alkylthiophene), regioregular poly(3-octylthiophene).
General Description
3-Octylthiophene is an alkyl thiophene derivative. It has been synthesized by the reaction of 3-bromothiophene with octylmagnesium bromide.
InChI:InChI=1/C12H20S/c1-2-3-4-5-6-7-8-12-9-10-13-11-12/h9-11H,2-8H2,1H3
New star-shaped non-fullerene acceptors (5Z,5′Z,5′′Z)-5,5′,5′′-((benzo[1,2-b : 3,4-b′ : 5,6-b′′]trithiophene-2,5,8-triyltris(4-octylthiophene-5,2-diyl))tris(methaneylylidene))tris(3-octyl-2-thioxothiazolidin-4-one) (1: BTT-OT-ORD) and 2,2′,2′′-((5Z,5′Z,5′′Z)-((benzo[1,2-b : 3,4-b′ : 5,6-b′′]trithiophene-2,5,8-triyltris(4-octylthiophene-5,2-diyl))tris(methaneylylidene))tris(3-octyl-4-oxothiazolidine-5,2-diylidene))trimalononitrile (2: BTT-OT-OTZDM) with a benzotrithiophene core, alkyl-thiophen units, and acceptor units were designed and synthesized. The HOMO-LUMO levels of 1 and 2 were determined by photoemission spectroscopy and UV-Vis absorption spectroscopy. Binary blend and ternary blend bulk heterojunction (BHJ) organic solar cells with non-fullerene acceptors 1 and 2 were fabricated with the inverted device structures of glass/ITO/ZnO/active_layer/MoO3/Ag. Both binary blend BHJ solar cells with 1 and 2 show lower JSC and larger VOC values than P3HT : PCBM solar cells. On the other hand, ternary blend BHJ organic solar cells, including 10 % of 1, exhibited a larger power conversion efficiency than P3HT : PCBM solar cells because the JSC value was largely improved.
Bolaamphiphilies with D-A-D type π-conjugated rigid cores composed by thiophenes as donors (D) and benzothiadiazole (BTD) as central acceptor (A) have been synthesised. Their self-assemblies and photophysical properties were investigated by polarising optical microscope, differential scanning calorimetry, X-ray diffraction and scanning electron microscopy. Such compounds can self-assemble into honeycomb cylinder mesophases with Colhex?/p6mm and Colsqu/p4mm lattices in their pure states as well as organogels with different morphologies in organic solvents. Their absorption spectra cover nearly the entire visible light range and their band gaps are relatively low. Tetrathiophene BTD based bolaamphilphiles (BT4/n) with higher D/A ratios than the bisthiophene BTD bolaamphilphiles (BT2/n) can self-assemble into more ordered nanostructures in both bulk states and solution. Both the absorption and emission peaks of BT4/n are strongly red shifted. The influence of the molecular conformation, the conjugated core length, as well as the D/A ratio on the self-assemble and photophysical characteristics of such D-A-D bolaamphiphiles are discussed.
Herein, the optical properties of thiophene-functionalized quadrupolar carbo-benzenes and a benzenic parent, of generic structure Th–C[tbnd]C–[core]–C[tbnd]C–Th, Th = R2C4HS, are comparatively investigated. Beyond the previously unknown dioctylthienylethynylbenzene (core = p-C6H4, R = nOct), two bis-dialkylthienylethynyl-carbo-benzenes (core = C18Ph4, R = nOct, nBu) are envisaged for the unique “carbo-aromatic” character of the C18 macrocycle. The three targets were synthesized from the corresponding ethynylthiophenes in 47, 20 and 10% yield, respectively, then characterized by classical methods such as NMR spectroscopy, and X-ray crystallography for one of the carbo-benzenes. Regarding linear and nonlinear optical properties, our results show that the carbo-merization induces a significant shift to lower energies of the one-photon electronic excitations accompanied by an 8-fold increase of the molar extinction coefficient compared to the parent molecule. Intriguingly, these excitations lead to a broad band of photoluminescence comprising decay transitions of the type S1 → S0 but also of the type S2 → S0. This phenomenon of emission from higher excited states, which is contrary to Kasha's rule, is assigned to - or revealed by - a reduction of the internal conversion efficiency between S2 and S1. Two-photon induced transitions are also enhanced, the two-photon absorption cross-section (σ2PA) being in average five times larger for the carbo-benzenes than for their benzene parent in the wavelength range 650–950 nm, with a maximum of σ2PA = 1430 GM (1 GM = 10?50 cm4 s/photon). Beyond a moderate nonlinearity, this comparative study provides quantitative insights about the way carbo-merization or insertion of a π-conjugated macrocycle between chromophoric functions (here thiophene rings) can tune optical properties of organic molecules. The optical properties of the bis-dialkylthienylethynyl-carbo-benzenes are also discussed in regard of recent reports on organic chromophores based on other types of π-conjugated macrocyclic cores.
The nickel(II) pincer complex, [NiCl(PhPNP)] (PhPNP = anion of 2,5-bis(diphenylphosphinomethyl)pyrrole), has been employed as a precatalyst for the Suzuki-Miyaura cross-coupling reaction of aryl halides and boronic acids. Both electron-rich and electron-deficient aromatic bromides were found to undergo coupling with boronic acids in modest yield at elevated temperature in the presence of K3PO4·H2O. Preliminary mechanistic studies of the reaction identified a novel species formulated as the boronate complex, [Ni(OB{OH}{2-tolyl})(PhPNP)], which most likely represents a catalyst deactivation pathway. The productive catalytic cycle was found to be most consistent with a Ni(I)/Ni(III) process where the boronic acid serves as both reductant and nucleophile in the presence of base.
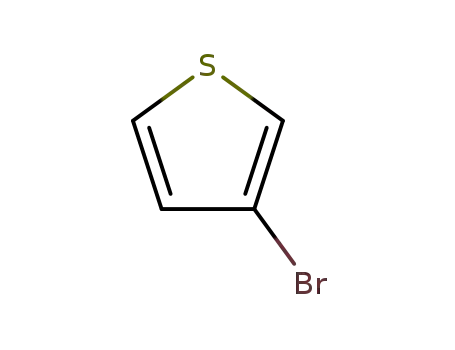
3-Bromothiophene

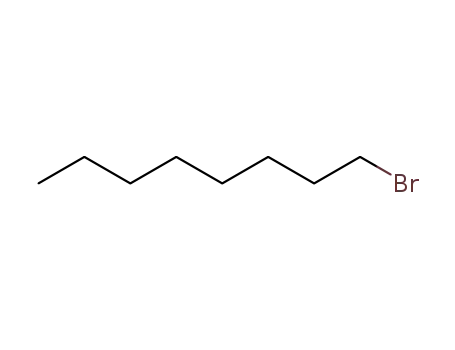
1-bromo-octane


3-octylthiophene
| Conditions | Yield |
|---|---|
|
1-bromo-octane;
With
magnesium;
In
diethyl ether;
3-Bromothiophene;
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
In
diethyl ether;
for 24h;
Further stages.;
Heating;
|
73% |
|
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
regioselective reaction;
|
73% |
|
1-bromo-octane;
With
magnesium;
In
diethyl ether;
for 2h;
Inert atmosphere;
3-Bromothiophene;
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
for 24h;
Cooling with ice;
|
68% |
|
3-Bromothiophene; 1-bromo-octane;
With
magnesium;
In
diethyl ether;
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
|
|
|
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II); magnesium;
In
diethyl ether;
|

3-Bromothiophene

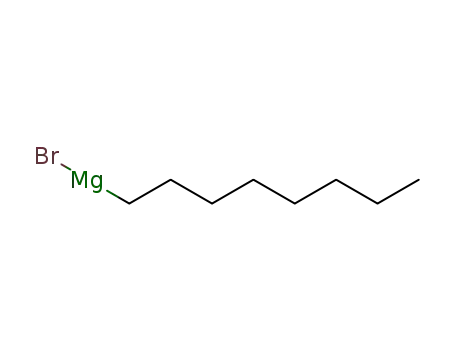
octylmagnesium bromide


3-octylthiophene
| Conditions | Yield |
|---|---|
|
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
In
diethyl ether;
at 0 - 5 ℃;
for 0.25h;
Inert atmosphere;
|
98.2% |
|
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
at 0 - 20 ℃;
Inert atmosphere;
|
88% |
|
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
In
tetrahydrofuran;
for 15h;
Reflux;
|
86% |
|
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
In
tetrahydrofuran;
at 20 ℃;
for 12h;
|
78% |
|
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
In
diethyl ether;
at 35 ℃;
|
61% |
|
nickel;
|
|
|
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
|
|
|
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
In
tetrahydrofuran;
for 15h;
Reflux;
|
|
|
With
1,2-bis(diphenylphosphino)ethane nickel(II) chloride;
In
diethyl ether;
at 0 ℃;
for 3h;
Inert atmosphere;
|
|
|
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
In
tetrahydrofuran;
Reflux;
|

3-Bromothiophene
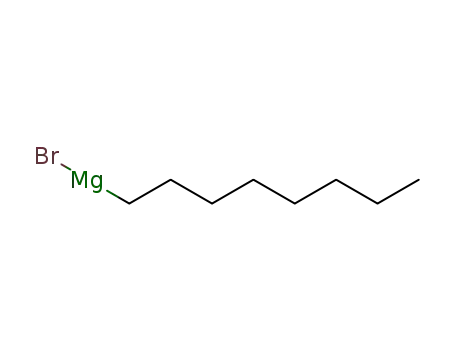
octylmagnesium bromide

1-bromo-octane
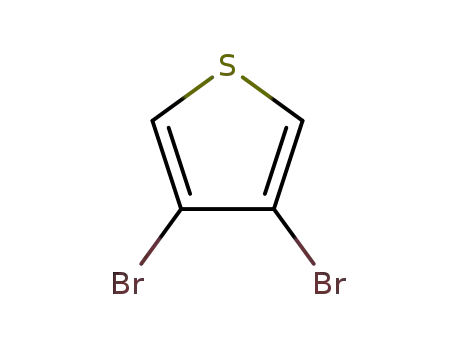
3,4-dibromothiophene
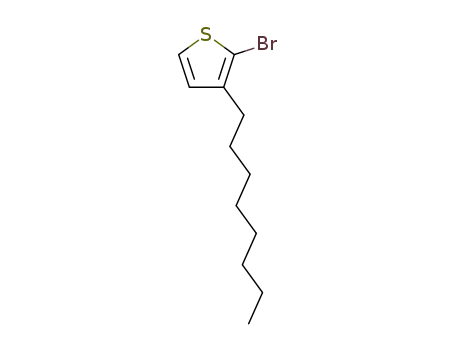
2-bromo-3-octylthiophene
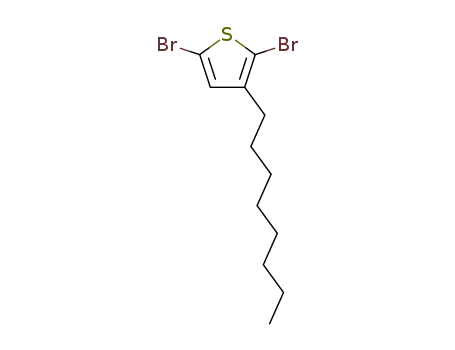
2,5-dibromo-3-octadecylthiophene

4,4'-dioctyl-2,2'-bithiophene
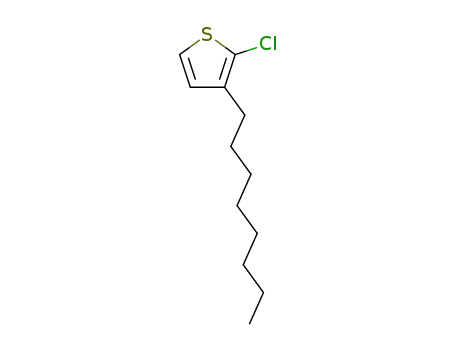
2-chloro-3-octylthiophene
CAS:139100-06-4
CAS:145543-83-5
CAS:144012-09-9