Your Location:Home >Products >OLED intermediates >Fluorenes >89827-45-2
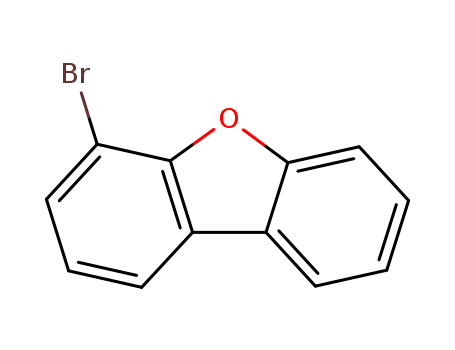

Product Details
Chemical Properties
White Crysstalline
Uses
Versatile scaffold to get entry to 4-substituted dibenzofuranes. Substituted dibenzofuranes have utility in semiconductors and OLED applications.
An efficient and practical method has been developed for the preparation of aryl bromides via the direct bromodeboronation of arylboronic acids with CuBr2 in water. This strategy provides several advantages, such as being ligand-free, base-free, high yielding, and functional group tolerant.
The invention provides a compound, an electronic component and an electronic device, and relates to the technical field of organic materials, wherein the compound is represented by a formula I, X1, X2and X3 are the same or different and are respectively and independently selected from carbon and nitrogen, X1, X2 and X3 are not carbon at the same time, L is selected from a single bond, arylene, heteroarylene, aryl alkylene and heteroaryl alkylene, Ar1 is selected from alkyl, cycloalkyl, aryl, heteroaryl, alkoxy, alkylamino, arylamino and arylalkylamino, and Ar2 and Ar3 are the same or different and are respectively and independently selected from cycloalkyl, aryl, heteroaryl, alkoxy, alkylamino, arylamino and arylalkylamino. The compound provided by the invention can reduce the working voltage of an electronic component, improve the luminous efficiency of the electronic component and prolong the service life of a device.
Combined use of oxorhenium catalysts with triphenyl phosphite as an oxygen acceptor allowed efficient deoxygenative aromatization of oxabicyclic dienes. The reaction proceeded under neutral conditions and was compatible with various functional groups. Combining this deoxygenation with regioselective bromination and trapping of the generated aryne with furan resulted in benzannulative π-extension at the periphery of the PAHs. This enabled direct use of unfunctionalized PAHs for extension of π-conjugation. Iteration of the transformations increased the number of fused-benzene rings one at a time, which has the potential to alter the properties of PAHs by fine-tuning the degree of π-conjugation, shape, and edge topology.
The present invention addresses the problem of providing a novel aromatic heterocyclic derivative, providing an organic EL element that uses the derivative and has high luminous efficiency, long luminescence life, and few changes over time when used under high temperatures, and providing a display device and an illumination device that are equipped with the organic El element. This aromatic heterocyclic derivative is characterized by having a specific structure which includes a carbazole ring.
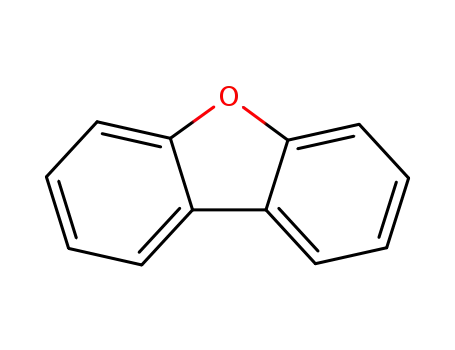
dibenzofuran

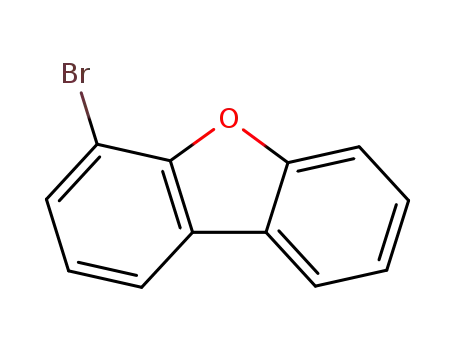
4-bromodibenzofuran
| Conditions | Yield |
|---|---|
|
dibenzofuran;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -40 - 20 ℃;
With
ethylene dibromide;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
|
76% |
|
dibenzofuran;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -70 - 0 ℃;
for 2h;
Inert atmosphere;
With
ethylene dibromide;
In
tetrahydrofuran; hexane;
for 12h;
|
75% |
|
dibenzofuran;
With
n-butyllithium;
In
tetrahydrofuran;
at -40 - 20 ℃;
for 2.5h;
Inert atmosphere;
With
ethylene dibromide;
In
tetrahydrofuran;
at -78 - 20 ℃;
for 2.5h;
|
74% |
|
dibenzofuran;
With
n-butyllithium;
In
tetrahydrofuran;
at -40 - 20 ℃;
for 2.5h;
Inert atmosphere;
With
ethylene dibromide;
In
tetrahydrofuran;
at -78 - 20 ℃;
for 2.5h;
|
74% |
|
dibenzofuran;
With
n-butyllithium;
In
tetrahydrofuran;
at -40 - 25 ℃;
for 2.5h;
Inert atmosphere;
With
1,1-dibromomethane;
at -78 - 25 ℃;
for 2.5h;
regioselective reaction;
Inert atmosphere;
|
68% |
|
dibenzofuran;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -30 - 20 ℃;
Inert atmosphere;
With
ethylene dibromide;
In
tetrahydrofuran; hexane;
at -60 - 20 ℃;
|
62% |
|
dibenzofuran;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -30 - 20 ℃;
for 6h;
Inert atmosphere;
With
ethylene dibromide;
In
tetrahydrofuran; hexane;
at -60 - 20 ℃;
for 16h;
|
62% |
|
With
n-butyllithium; diethyl ether; bromine;
|
|
|
With
n-butyllithium;
Yield given. Multistep reaction;
1,) THF, hexane, 25 deg C, 5 h; 2.) THF, 25 deg C, 2 h;
|
|
|
With
n-butyllithium; ethylene dibromide;
Yield given. Multistep reaction;
1.) hexane, 25 deg C, 5 h; 2.) THF, RT, 2 h;
|
|
|
dibenzofuran;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -30 - 20 ℃;
for 6h;
With
ethylene dibromide;
In
tetrahydrofuran; hexane;
at -60 - 20 ℃;
for 16h;
|
|
|
dibenzofuran;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -65 - 20 ℃;
for 3h;
Inert atmosphere;
Heating;
With
ethylene dibromide;
In
tetrahydrofuran; hexane;
at -65 - 20 ℃;
for 3h;
Heating;
|
|
|
dibenzofuran;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -30 - 20 ℃;
for 6h;
Inert atmosphere;
With
ethylene dibromide;
In
tetrahydrofuran; hexane;
at -60 - 20 ℃;
for 16h;
Inert atmosphere;
|
|
|
dibenzofuran;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at 20 ℃;
for 6h;
Inert atmosphere;
With
ethylene dibromide;
In
tetrahydrofuran; hexane;
at -60 - 20 ℃;
for 16h;
|
|
|
dibenzofuran;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -30 - 20 ℃;
Inert atmosphere;
With
ethylene dibromide;
In
tetrahydrofuran; hexane;
at -60 - 20 ℃;
Inert atmosphere;
|
|
|
dibenzofuran;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -30 - 20 ℃;
for 6h;
Inert atmosphere;
With
ethylene dibromide;
In
tetrahydrofuran; hexane;
at -60 - 20 ℃;
for 16h;
|
70 g |
|
With
n-butyllithium; 1,1-dibromomethane;
In
tetrahydrofuran;
at -75 ℃;
|
|
|
With
n-butyllithium; ethylene dibromide;
In
tetrahydrofuran;
at -75 ℃;
|
|
|
dibenzofuran;
With
n-butyllithium;
In
tetrahydrofuran;
With
ethylene dibromide;
In
tetrahydrofuran;
|
|
|
dibenzofuran;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 - 0 ℃;
for 6h;
Inert atmosphere;
With
ethylene dibromide;
In
tetrahydrofuran;
at 20 ℃;
for 10h;
|
|
|
dibenzofuran;
With
n-butyllithium;
at -40 ℃;
With
ethylene dibromide;
at -78 ℃;
|
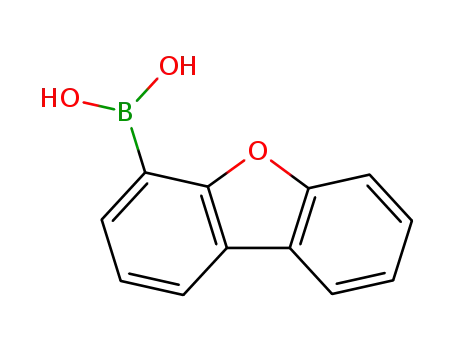
4-dibenzofurylboronic acid


4-bromodibenzofuran
| Conditions | Yield |
|---|---|
|
With
tetrabutylammomium bromide; copper(ll) bromide;
In
water;
at 100 ℃;
Sealed tube;
|
89% |

dibenzofuran
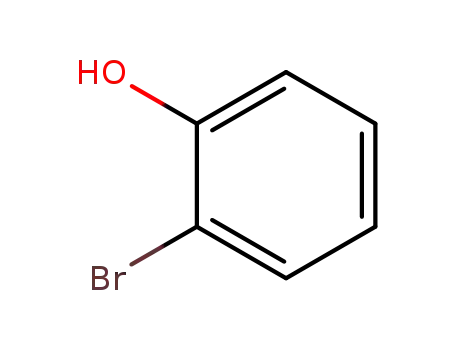
2-hydroxybromobenzene
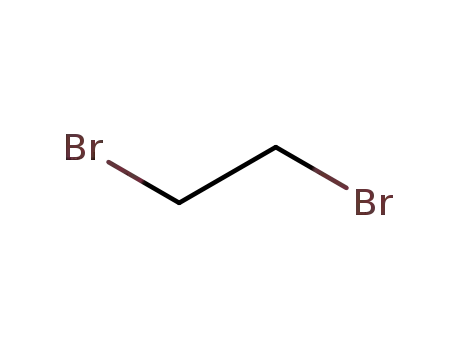
ethylene dibromide
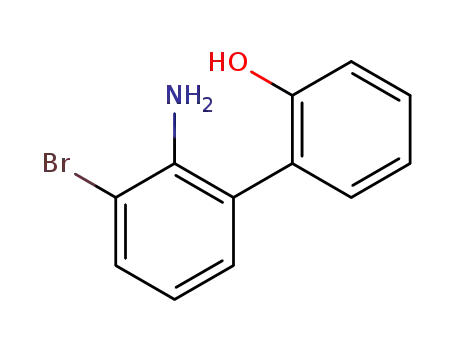
2′-amino-3′-bromo-[1,1′-biphenyl]-2-ol
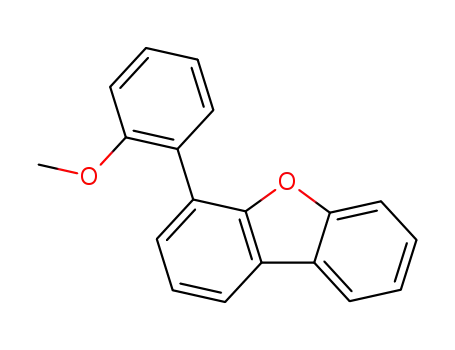
4-(2-methoxyphenyl)dibenzofuran
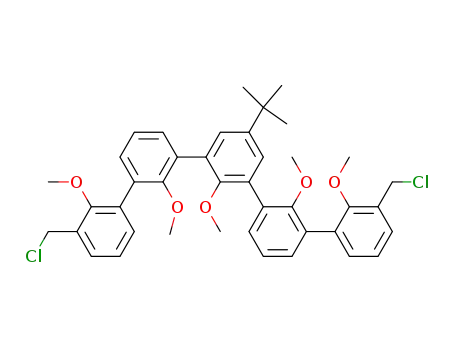
3,3-bis(chloromethyl)-5-(1,1-dimethylethyl)-2,2',2,2',2-pentamethoxy-<1,1':3',1:3,1':3',1-quinquephenyl>
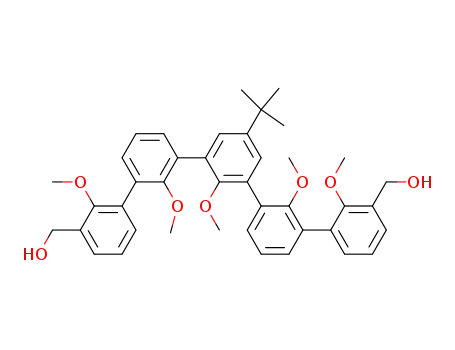
5-(1,1-dimethylethyl)-2,2',2,2',2-pentamethoxy<1,1':3',1:3,1':3',1-quinquephenyl>-3,3-dimethanol
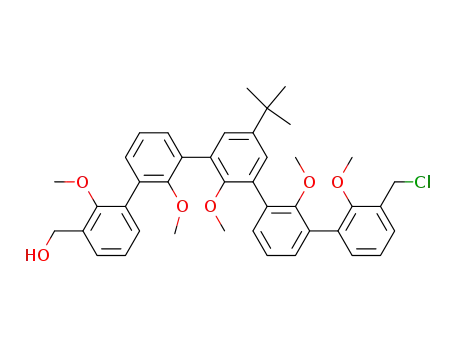
5-(1,1-dimethylethyl)-3-(chloromethyl)-3-(hydroxymethyl)-2,2',2,2',2-pentamethoxy-<1,1':3',1:3,1':3',1-quinquephenyl>
CAS:1548450-59-4
CAS:128363-76-8
CAS:97511-05-2