Your Location:Home >Products >OLED intermediates >Boric acids >164461-18-1
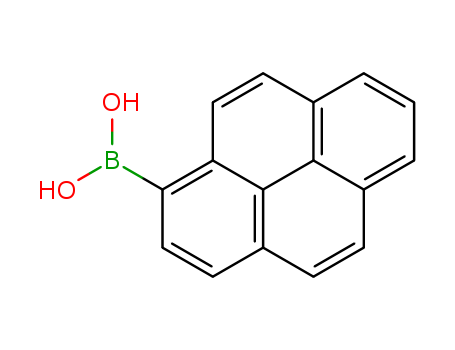

Product Details
Chemical Properties
White to light brown solid
Uses
It is an important raw material and intermediate used in organic synthesis agrochemical, pharmaceutical and dyestuff field. Also used as a intermediate for organic light-emitting diode(OLED).
Uses
suzuki reaction
InChI:InChI=1/C16H11BO2/c18-17(19)14-9-7-12-5-4-10-2-1-3-11-6-8-13(14)16(12)15(10)11/h1-9,18-19H
The nucleoside 5-(1-pyrenyl)-2′-deoxyuridine (1) was prepared by a Suzuki-Miyaura cross-coupling reaction and subsequently used as a DNA building block in order to prepare a range of modified oligonucleotides using phosphoramidite chemistry. The DNA duplexes contain a pyrenyl group covalently attached to the nucleobase uracil. Upon excitation at 340 nm an intramolecular electron transfer from the pyrenyl group to the uracil moiety takes place which represents an injection of an excess electron into the DNA base stack. Based on the results obtained by steady-state fluorescence and time-resolved pump-probe laser spectroscopy it was possible to show that base-to-base electron transfer can occur from the Py-dU group only to adjacent thymines.
This work presents a fast purification system that involves a vertical quartz tube and vacuum sublimation for separation without dilution. This system produced re-crystallized 1,4-di(pyren-1-yl)benzene (DPB) in a few hours following the synthesis of pyrene derivatives with a mole yield ratio of 1,4-di(pyrene-1-yl)benzene to 1-phenylpyrene (1PP) of 5:1. Differential scanning calorimetry (DSC) and X-ray diffractometry (XRD) were utilized to identify the products. Finally, the lifetimes of pyrene derivatives DPB and 1PP were determined by exponential regression to be 10.0 ± 0.14 and 3.3 ± 0.15 ns, respectively. From the lifetime measurements, the purity of the photo-responsive materials could be easily determined. To prevent red-shift of electro-luminescence (EL) spectra by the energy transfer mechanism, it has acquired to elect transfer layers for pure electroluminescence. Aco-catalytic reaction reduced the amount of impurities and eliminated the need for the use of a solvent in the purification step.
Two pyrene boronic acid cyclic esters, PPB and NPB, were prepared and their solid state fluorescence properties were investigated. Interestingly, the results showed that PPB with a 5-membered ring possessed reversible mechanoluminescence and vapochromic behaviour with a fast self-recovering ability at room temperature, whereas NPB with a 6-membered ring did not. It was demonstrated that the mechanically responsive fluorescence properties of PPB and NPB were highly related to the molecular packing mode and the solid state plasticity.
Solid-state emissive dyes with mechanochromic luminescence (MCL) properties change their emission colors upon exposure to mechanical stimuli. During the recent rapid advances in the area of MCL, considerable efforts have been focused on elucidating the MCL behavior of single-component dyes that can switch their solid-state emission between two colors. Herein, a novel strategy to achieve tricolor MCL is presented, which is based on mixing two organic dyes that exhibit poor MCL or no MCL properties, respectively. We propose that the tricolor MCL of the two-component dye should be attributed to the transition of the fraction with poor MCL properties from a crystalline to two amorphous states, specifically a perfectly amorphous and a proto-crystalline amorphous state. The present system should have advantages in terms of the availability of MCL dyes and the variety of potential combinations of two-component dyes. By making a use of the underlying design principle developed in this study, a wide variety of new MCL systems should be accessible, which should also accelerate the investigation of practical applications of MCL dyes.
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous environmental pollutants. Due to its structural similarity with the potent carcinogen dibenzo [a,l] pyrene (DB [a,l]P) and because of its environmental presence, dibenzo[c,mno]chrysene (naphtho[1,2-a]pyrene, N[1,2-a]P) is of considerable research interest. We therefore developed an efficient synthesis of N[1,2-a]P, and examined its in vitro metabolism by male Sprague Dawley rat liver S9 fraction. Its mutagenic activity in S. typhimurium TA 100 and its morphological cell transforming ability in mouse embryo fibroblasts were evaluated. On the basis of spectral analyses, the in vitro major metabolites were identified as the fjord region dihydrodiol trans-9,10-dihydroxy-9,10- dihydro-N[1,2-a]P (N[1,2-a]P-9,10-dihydrodiol), the K-region diols N[1,2-a]P-4,5-dihydrodiol and N[1,2-a]P-7,8-dihydrodiol, and also the 1-, 3-, and 10-hydroxy-N[1,2-a]P; the structure of N[1,2-a]P-9,10-dihydrodiol was also confirmed by independent synthesis. In assays with S. typhimurium TA 100, N[1,2-a]P-9,10-dihydrodiol was half as mutagenic as (±)-trans-7,8-dihydroxy-7,8-dihydrobenzo[a]pyrene (B[a]P-7,8-dihydrodiol) at ≥ nmol/plate. N[1,2-a]P-9,- 10-dihydrodiol was much more mutagenic than N[1,2-a]P at all dose levels, suggesting that the N[1,2-a]P-9,10-dihydrodiol is the likely proximate mutagen of N[1,2-a]P. Evaluation of morphological cell transformation in C3H10T1/2C18 mouse embryo fibroblasts revealed that N[1,2-a]P was comparable to B[a]P. We further examined the pattern of in vitro adduct formation between calf thymus DNA and (±)-anti-9,10-dihydroxy-9,10-dihydro-11,12-epoxy- 9,10,11,12-tetrahydro-N[1,2-a]P (N[1,2-a]PDE) and found that dG-adduct formation is 2.9-fold greater than dA-adduct formation. On the basis of our results and those reported in the literature, our working hypothesis is that N[1,2-a]P may be added to the list of potent carcinogens that includes DB [a,l]P. This hypothesis is currently being tested in our laboratory.
The synthesis and photophysical properties of two Ru(II) diimine complexes bearing one (dyad) and three (tetrad) pyrenyl units, respectively, are presented. The pyrene chromophore in each metal complex is tethered through a single C-C bond in the 5-position of 1,10-phenanthroline (py-phen). Both Ru(II) complexes display increased absorption cross sections near 340 nm largely due to the presence of the pyrenyl chromophore(s). Excitation from 300 to 540 nm results exclusively in the observation of metal-to-ligand charge transfer (MLCT) based emission that is exceptionally long lived, 23.7 μs and 148 μs in deaerated CH3CN, respectively. This luminescence was analyzed using steady-state and time-resolved techniques at room temperature and 77 K. The tetrad complex, [Ru(py-phen)3]2+, displays a dynamic self-quenching reaction at room temperature in dilute CH3CN solutions that is well modeled by a Stern-Volmer expression. The excited-state processes occurring between the MLCT core and the pyrenyl units were further evaluated with ultrafast transient absorption spectroscopy and conventional flash photolysis. Formation of the 3pyrene absorption was directly monitored in both complexes and ranged from 2.8 × 1010 s-1 in [Ru(bpy)2(py-phen)]2+ to 2.4 × 1011 s-1 in [Ru(py-phen)3]2+. In both cases, the transient absorption spectra contain features of 3pyrene excited states, whereas the room-temperature luminescence is MLCT-based, both decaying with the same kinetics. This is consistent with the formation of a thermal excited-state equilibrium between the two triplet states at room temperature. Both Ru(II) complexes were found to sensitize the production of molecular singlet oxygen with a quantum efficiency of 0.69, measured by observing the characteristic 1O2 luminescence at 1270 nm.
Polymer light-emitting diodes (PLEDs) have attracted much attention from academia and industry field because PLED has advantage property that is well-suited to flexible lighting and solution processed device. In this paper, we suggest new blue emitting polymer based on pyrene, poly(9-(3-Vinyl-phenyl)-pyrene) (PVPPy). From NMR data, vinyl group protons were disappeared and aromatic protons showed broad proton peaks because of polymer characteristics. PVPPy film can be obtained from spin coating with solution used by common solvents. It exhibited photoluminescence (PL) maximum value of 468 nm and full width at half maximum (FWHM) value of 73 nm. Three dopants for green, red, yellow were used to PVPPy, all energy transfer was happened well.
Controlling the behavior of mechanochromic luminescence (MCL) based on rational design principles is an outstanding challenge. Herein, an unprecedented self-recovering MCL that manifests in a large shift of the emission maximum (~200 nm) has been achieved for 2-alkyl-4-(pyren-1-yl)thiophenes upon introducing long alkyl chains and mixing with N,N′-dimethylquinacridone.
We describe how the morphology, photoluminescence (PL), and electroluminescence properties of 2,2′,7,7′-tetrakis(pyren-1-yl)-9, 9′-spirobifluorene (TPSBF) are related to its film thickness and how, under optimized conditions, the three main PL emissions constitute a form of white light. We fabricated high-brightness, broad-spectrum, white-light organic light-emitting diodes, incorporating a single emission layer of the blue-light-emitting material TPSBF, which comprises four pyrene units linked to a spirobifluorene core. An organic light-emitting device with the configuration indium tin oxide (170a nm)/4,4′,4′′-tris[N-(2-naphthyl)-N- phenylamino]triphenylamine (15a nm)/4,4′-bis[N-(1-naphthyl)-N-phenylamino] biphenyl (65a nm)/TPSBF (50a nm)/tris(8-hydroxyquinoline)aluminum (30a nm)/LiF (0.8a nm)/Al (200a nm) exhibited a broad-spectrum white emission with a maximum luminescence and current efficiency of 57 680a cd m-2 and 6.51a cd A-1, respectively, and Commission International De l'Eclairage coordinates of (0.29,0.36). All aglow: High-brightness, broad-spectrum, white-light organic light-emitting diodes, incorporating a single emission layer of the blue-light-emitting material 2,2′,7,7′-tetrakis(pyren-1-yl)- 9,9′-spirobifluorene (TPSBF), which comprises four pyrene units linked to a spirobifluorene core, are described (see figure). Copyright
Pyrene molecules containing NBN-doped polycyclic aromatic hydrocarbons (PAHs) have been synthesized by a simple and efficient intermolecular dehydration reaction between 1-pyrenylboronic acid and aromatic diamine. Pyrene-B (o-phenylenediamine) with a five-membered NBN ring and pyrene-B (1,8-diaminonaphthalene) with a six-membered NBN ring show differing luminescence. Pyrene-B (o-phenylenediamine) shows concentration-dependent luminescence and enhanced emission after grinding at solid state. Pyrene-B (1,8-diaminonaphthalene) exhibits a turn-on type luminescence upon fluoride ion addition at lower concentration, as well as concentration-dependent stability. Further potential applications of Pyrene-B (o-phenylenediamine) on artificial light-harvesting film were demonstrated by using commercial NiR dye as acceptor.
The present invention relates to a compound having a cyclic substituent fused with a cyclic ring and an organic light emitting diode including the same, and more particularly, to a compound for an organic light emitting diode represented by chemical formula A and an organic light emitting diode including the same. In chemical formula A, X is a substituent having structural formula X, Y is a substituent of structural formula Y1, n is an integer from 1 to 4, and structural formulas X and Y1 are the same as described in detailed description of the present invention.
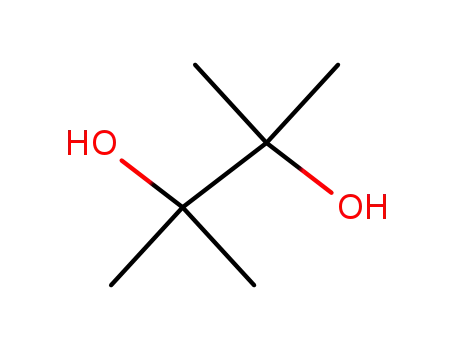
2,3-dimethyl-2,3-butane diol

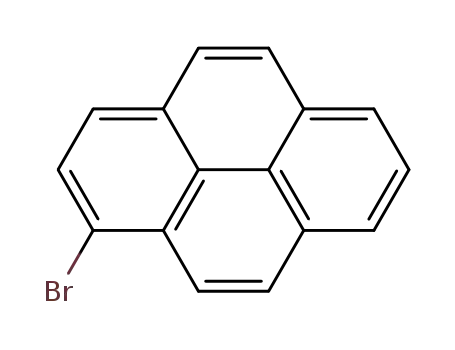
1-bromopyrene

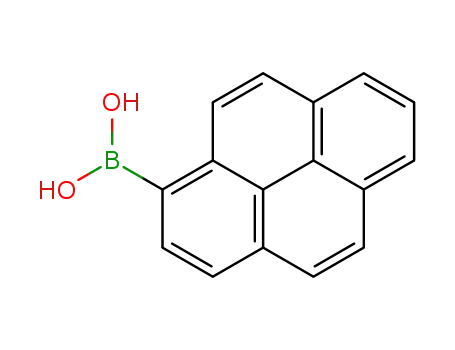
1-pyrenylboronic acid

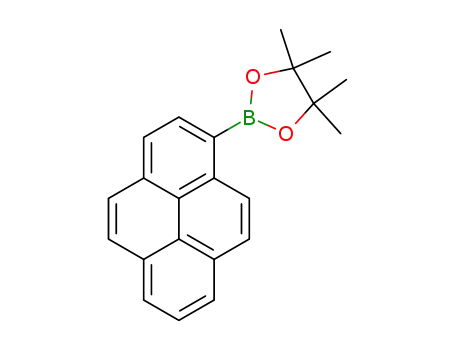
1-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrene
| Conditions | Yield |
|---|---|
|
1-bromopyrene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 0.5h;
With
Triisopropyl borate;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
for 0.5h;
2,3-dimethyl-2,3-butane diol;
In
diethyl ether; hexane;
at 40 ℃;
for 0.75h;
|
85% |
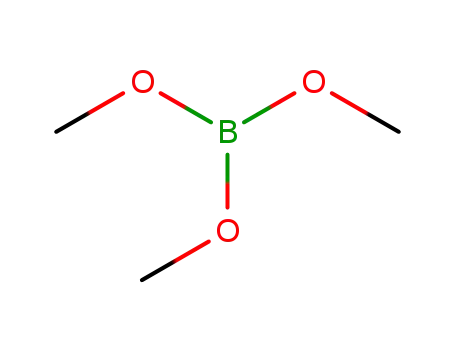
Trimethyl borate

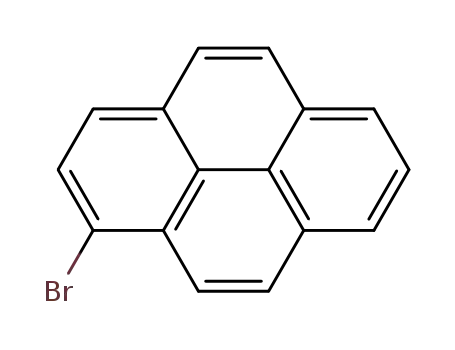
1-bromopyrene


1-pyrenylboronic acid
| Conditions | Yield |
|---|---|
|
1-bromopyrene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 1h;
Trimethyl borate;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
for 2h;
|
74% |
|
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
for 3h;
|
74% |
|
1-bromopyrene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 - 0 ℃;
for 1h;
Inert atmosphere;
Trimethyl borate;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
for 24h;
With
hydrogenchloride;
In
tetrahydrofuran; hexane;
for 1h;
|
70% |
|
With
hydrogenchloride; n-butyllithium;
In
diethyl ether; hexane;
BuLi soln. (hexane) addn. to org. compd. soln. (Et2O) at 0°C, mixt. addn. to B-compd. soln. (Et2O) at -78°C over 30 min, stirring 3 h at -50 to -70°C and 60 h at room temp., 2 M HCl addn., stirring 2 h, org. phase sepn.; org. phase washing (water), drying (MgSO4), treating with charcoal, ethereal soln. concn. to dryness, residue vac. drying at 50°C;
|
61% |
|
1-bromopyrene;
With
n-butyllithium;
In
diethyl ether;
Trimethyl borate;
|
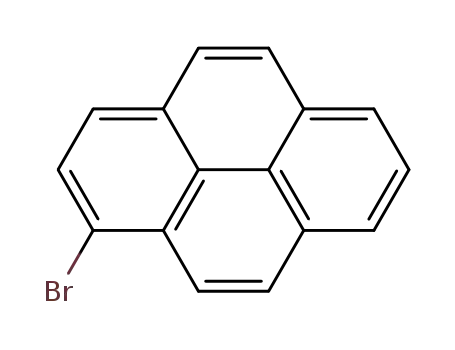
1-bromopyrene

2,3-dimethyl-2,3-butane diol

Trimethyl borate
oxonium
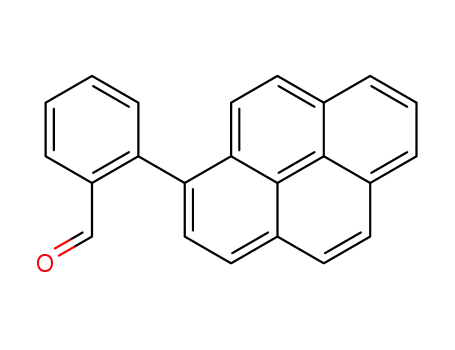
2-(1-Pyrenyl)benzaldehyde
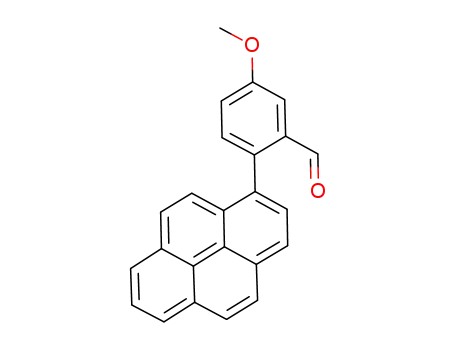
1-(2-formyl-4-methoxyphenyl)pyrene
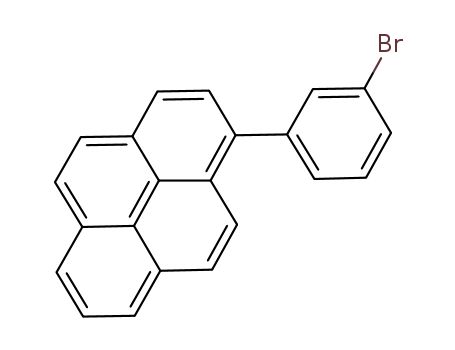
1-(3-bromophenyl)pyrene
1-(3,5-dibromophenyl)pyrene
CAS:867374-53-6
CAS:1259280-37-9
CAS:854952-58-2