Your Location:Home >Products >OLED intermediates >Boric acids >854952-58-2
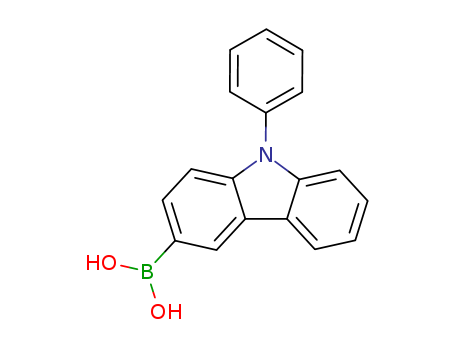

Product Details
Uses
9-Phenyl-9H-carbazol-3-ylboronic acid is suzuki reaction
Uses
9-Phenyl-9H-carbazol-3-ylboronic acidis used in the synthesis of alkyl-functionalized organic dyes.
Uses
B-(9-Phenyl-9H-carbazol-3-yl)boronic Acid is used in the synthesis of alkyl-functionalized organic dyes.
Three host materials, DCzPh, DCzPy and DCzPm, were developed for phosphorescent organic light emitting devices (PhOLEDs). These three compounds exhibit high triplet energy level (ET > 2.7 eV), suitable glass transition temperatures (Tg > 130 °C), appropriate highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) levels, and balanced charge-transporting properties. Green PhOLEDs were fabricated by utilizing these three materials as hosts and the efficiencies were satisfactory. Device G3 based on DCzPm exhibited high efficiency with the maximum external quantum efficiency (EQE) of 17.2%, and even at the brightness of 1000 cd m?2, the EQE reached 17.1%, demonstrating its low efficiency roll-off. The above results indicate that these host materials possess great commercial potential in OLED applications.
An object of the present invention is to provide a compound capable of improving high luminous efficiency, low driving voltage and lifespan of a device, an organic electric element using the same, and an electronic device thereof. The present invention provides the compound represented by any one of chemical formulas 6 to 9. By using the compound according to the present invention, high luminous efficiency and low driving voltage of the device can be achieved, and the lifespan of the device can be greatly improved.
The invention discloses a preparation method of N-arylcarbazole-3-boric acid, and belongs to the field of liquid crystal intermediates. The preparation method comprises the following steps: coupling carbazole with aryl halide in the presence of alkali to generate N-arylcarbazole, enabling the N-arylcarbazole to react with a bromination reagent to generate Naryl-3, 6-dibromo carbazole, enabling theNaryl-3, 6-dibromo carbazole to react with borate and butyl lithium by a one-pot method, and carrying out hydrolyzing to obtain N-arylcarbazole-3-boric acid. According to the method, dibromides whichare easy to purify are generated during bromination, monosubstituted products are generated by controlling the using amount of the lithiation reagent and the boric acid ester during lithiation, the method is verified on the scale of dozens of kilograms, and the method has the prospect of industrial methods.
Disclosed are an amine-based compound and an organic light emitting device including the same. The amine-based compound is represented by chemical formula 1A.
The present invention relates to an organic compound and an organic electroluminescent device and an electronic device using the same, wherein the organic compound has a structure formed by combining an indole carbazole-based planar conjugated group in a fused manner and a dibenzofuran substituted with an aryl group. The structure has higher hole mobility and higher energy transfer efficiency, and is suitable for being used as a light-emitting host material in an organic electroluminescent device. When the organic compound is used as a light emitting host material in an organic electroluminescent device, the light emitting efficiency and the service life of the device can be effectively improved, and the working voltage is reduced.
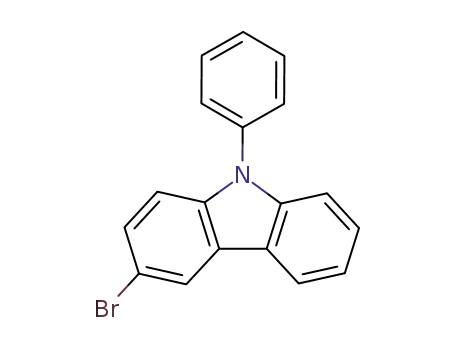
3-bromo-9-phenyl-9H-carbazole

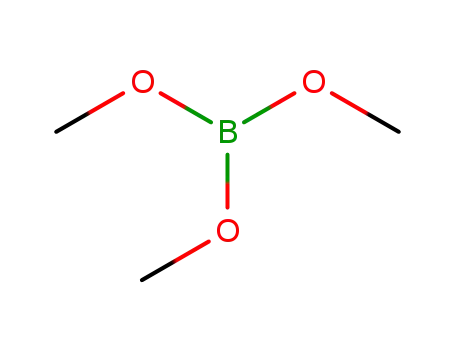
Trimethyl borate

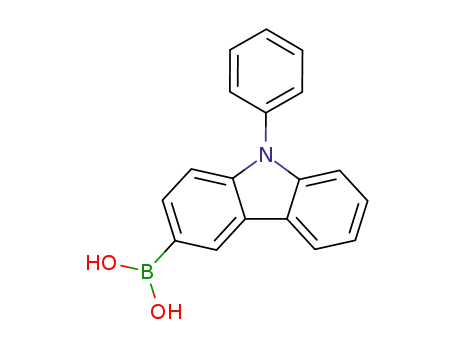
N-phenyl-9H-carbazol-3-boronic acid
| Conditions | Yield |
|---|---|
|
3-bromo-9-phenyl-9H-carbazole;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -80 ℃;
for 1h;
Trimethyl borate;
In
tetrahydrofuran; hexane;
at -80 - 20 ℃;
for 15h;
With
hydrogenchloride; water;
In
tetrahydrofuran; hexane;
at 20 ℃;
for 1h;
|
86% |
|
3-bromo-9-phenyl-9H-carbazole;
With
n-butyllithium;
In
tetrahydrofuran;
at -80 ℃;
Inert atmosphere;
Trimethyl borate;
In
tetrahydrofuran;
at -80 - 20 ℃;
With
hydrogenchloride; water;
In
tetrahydrofuran;
at 20 ℃;
for 1h;
|
86% |
|
With
hydrogenchloride; n-butyllithium; magnesium sulfate;
In
tetrahydrofuran; hexane; chloroform;
|
80% |
|
3-bromo-9-phenyl-9H-carbazole;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 2h;
Trimethyl borate;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
for 24h;
With
hydrogenchloride; water;
In
tetrahydrofuran; hexane;
at 20 ℃;
for 1h;
|
80% |
|
3-bromo-9-phenyl-9H-carbazole;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 2h;
Trimethyl borate;
In
tetrahydrofuran; hexane;
at 20 ℃;
for 24h;
With
hydrogenchloride;
In
water;
at 20 ℃;
for 1h;
|
80% |
|
3-bromo-9-phenyl-9H-carbazole;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 - 0 ℃;
for 1h;
Inert atmosphere;
Trimethyl borate;
In
tetrahydrofuran;
at -78 - 20 ℃;
for 12h;
Inert atmosphere;
|
75% |
|
3-bromo-9-phenyl-9H-carbazole;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 - 0 ℃;
for 1h;
Inert atmosphere;
Trimethyl borate;
In
tetrahydrofuran;
at -78 - 20 ℃;
for 12h;
Inert atmosphere;
|
75% |
|
3-bromo-9-phenyl-9H-carbazole;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 0.5h;
Trimethyl borate;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
for 1h;
With
hydrogenchloride; water;
In
tetrahydrofuran; hexane;
for 0.166667h;
|
69.82% |
|
3-bromo-9-phenyl-9H-carbazole;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -80 ℃;
for 1h;
Trimethyl borate;
In
tetrahydrofuran; hexane;
at 20 ℃;
for 15h;
With
hydrogenchloride; water;
In
tetrahydrofuran; hexane;
at 20 ℃;
for 1h;
|
68% |
|
3-bromo-9-phenyl-9H-carbazole;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 1h;
Trimethyl borate;
In
tetrahydrofuran;
at 78 ℃;
for 1h;
With
hydrogenchloride; water;
In
tetrahydrofuran;
at 20 ℃;
for 1h;
|
60% |
|
3-bromo-9-phenyl-9H-carbazole;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 1.5h;
Inert atmosphere;
Trimethyl borate;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
for 4.5h;
|
43% |
|
3-bromo-9-phenyl-9H-carbazole;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -73 ℃;
for 1h;
Inert atmosphere;
Trimethyl borate;
In
tetrahydrofuran; hexane;
for 4h;
Inert atmosphere;
|
4.6 g |
|
3-bromo-9-phenyl-9H-carbazole;
With
n-butyllithium;
at -78 ℃;
Trimethyl borate;
at -78 ℃;
With
hydrogenchloride;
In
water;
|

3-bromo-9-phenyl-9H-carbazole

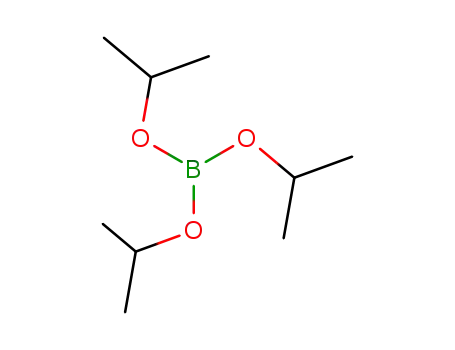
Triisopropyl borate


N-phenyl-9H-carbazol-3-boronic acid
| Conditions | Yield |
|---|---|
|
3-bromo-9-phenyl-9H-carbazole;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 3h;
Inert atmosphere;
Triisopropyl borate;
In
tetrahydrofuran; hexane;
at 20 ℃;
for 0.5h;
Inert atmosphere;
|
80% |
|
3-bromo-9-phenyl-9H-carbazole;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 2h;
Inert atmosphere;
Triisopropyl borate;
In
tetrahydrofuran;
at -78 - 20 ℃;
Inert atmosphere;
With
hydrogenchloride; water;
In
tetrahydrofuran;
at -10 ℃;
Inert atmosphere;
|
70% |
|
3-bromo-9-phenyl-9H-carbazole;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 1h;
Triisopropyl borate;
In
tetrahydrofuran; hexane;
at 20 ℃;
for 12h;
With
water;
In
tetrahydrofuran; hexane;
|
67.33% |
|
3-bromo-9-phenyl-9H-carbazole;
With
n-butyllithium;
In
diethyl ether; hexane;
at -78 ℃;
for 0.5h;
Triisopropyl borate;
In
diethyl ether; hexane;
at -78 - 20 ℃;
|

3-bromo-9-phenyl-9H-carbazole

Trimethyl borate
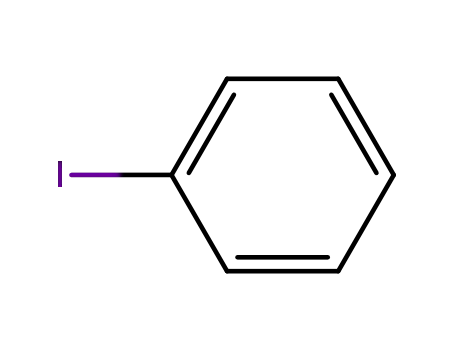
iodobenzene
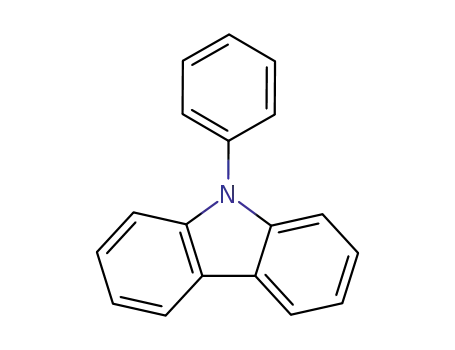
N-phenylcarbazole
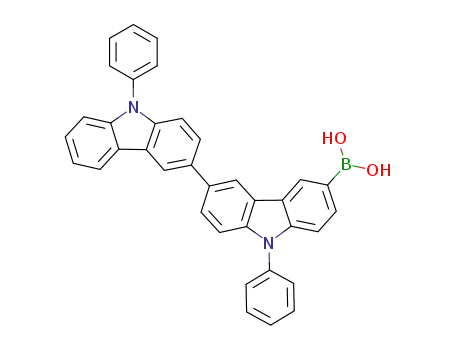
(9,9'-diphenyl-9H,9'H-[3,3'-biscarbazol]-6-yl)boronic acid
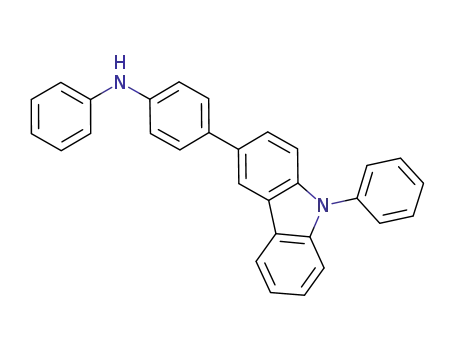
N-phenyl-N-[4-(9-phenylcarbazole-3-yl)phenyl]amine
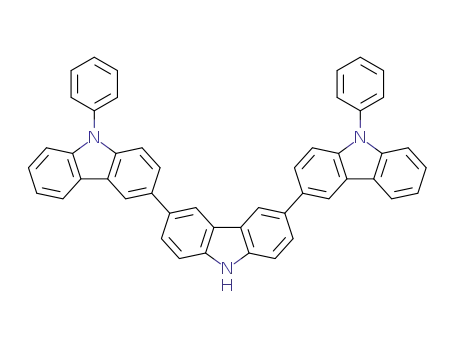
9,9″-diphenyl-9H,9′H,9″H-3,3':6′,3″-tercarbazole
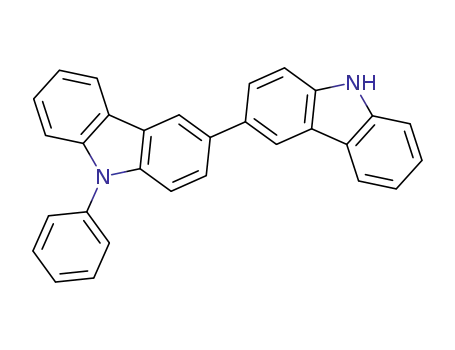
9-phenyl-3,3'-bicarbazole
CAS:65016-62-8
CAS:99586-26-2
CAS:1028648-22-7
Molecular Formula:C24H18BNO2
Molecular Weight:363.2
CAS:164461-18-1