Your Location:Home >Products >OLED intermediates >Carbazoles >50-00-0
Product Details
General Description
Formaldehyde, also called formic aldehyde or methyl aldehyde, has extensive application. For instance, it is used as a tissue preservative or organic chemical reagent. Thus, formaldehyde is very common to the chemical industry. In fact, formaldehyde is an important chemical used widely by industry to manufacture building materials and numerous household products. It is also a by-product of combustion and certain other natural processes. It is present in substantial concentrations both indoors and outdoors. Formaldehyde is well known as a preservative in medical laboratories, as an embalming fluid, and as a steriliser. Its primary use is in the production of resins and as a chemical intermediate. Urea–formaldehyde (uf) and phenol–formaldehyde (pf) resins are used in foam insulations, as adhesives in the production of particle board and plywood, and in the treating of textiles. Sources of formaldehyde in the home include building materials, smoking, household products, and the use of unvented, fuel-burning appliances, like gas stoves or kerosene space heaters. Formaldehyde, by itself or in combination with other chemicals, serves a number of purposes in manufactured products. Formaldehyde itself is a colourless gas, but it is more commonly purchased and used in aqueous solution (called formalin solution), with a maximum concentration of 40%. Formalin solutions often contain some amount of methanol as well. Both formaldehyde gas and solutions have a characteristic pungent, unpleasant odour.
Chemical Properties
Formaldehyde is colorless gas with a very distinct, pungent odor. It is highly soluble in water and in a variety of organic solvents. It has the potential to react explosively with peroxides and nitrogen oxide. Formalin, the aqueous form of formaldehyde, is a colorless liquid with a very distinct, pungent odor. It is incompatible and may react with strong oxidizers, alkalis,and acids. The liquid has a variable molecular weight, which is dependent on the specific aqueous formulation.
Uses
Formaldehyde (methyl aldehyde, methylene oxide) is a ubiquitous compound found endogenously in the body and environment. It is a colorless, flammable gas with a distinct, pungent odor and is most commonly available in aqueous solutions under the name formalin (37% formaldehyde in water). Formaldehyde has been used as a disinfectant, an embalming agent, and in industry as a precursor in the fabrication of complex compounds. Since scientific research has identified links between formaldehyde and adverse health effects, precautions and protections must be considered during use.
Description
Formaldehyde is a colorless, flammable gas with a distinctive pungent odor. It is the simplest aldehyde, which is a class of organic compounds with the carbonyl group bonded to at least one hydrogen atom. Formaldehyde was described by August Wilhelm von Hoff mann (1818–1892) in 1867 after the Russian Aleksandr Butlerov (1828–1886) had inadvertently synthesized it in 1857. Formaldehyde readily dissolves in water to produce a solution called formalin, which is commonly marketed as a 37% solution.
Chemical Properties
Formalin is made slightly alkaline (pH 8) by the addition of sodium hydroxide and then urea is added to give a urea to formaldehyde ratio of about 1: 2 molar. The resulting solution is boiled under reflux for about 15 minutes, acidified (to pH 4) with formic acid and then boiled for a further 5-20 minutes until the required degree of reaction is attained.
Chemical Properties
Formaldehyde is an important chemical widely used by industry to manufacture building materials and numerous household products. It is also a by-product of combustion and certain other natural processes. It is present in substantial concentrations both indoors and outdoors. Formaldehyde is well known as a preservative in medical laboratories, as an embalming fl uid, and as a sterilizer. Its primary use is in the production of resins and as a chemical intermediate. Urea formaldehyde (uf) and phenol formaldehyde (pf) resins are used in foam insulations, as adhesives in the production of particle board and plywood, and in the treating of textiles. Sources of formaldehyde in the home include building materials, smoking, household products, and the use of unvented, fuel-burning appliances, like gas stoves or kerosene space heaters. Formaldehyde, by itself or in combination with other chemicals, serves a number of purposes in manufactured products. It has been reported that the use and production of formaldehyde in 1998 was about 11.3 billion pounds and the international production crossed over 46 billion pounds in 2004.
Physical properties
Formaldehyde is a clear, colorless liquid with a pungent, suffocating odor. Burning taste. Experimentally determined odor threshold concentrations of 1.0 ppmv and 0.50 ppmv were reported by Leonardos et al. (1969) and Nagata and Takeuchi (1990), respectively. Also,formalin is an aqueous solution that is 37% formaldehyde by weight; inhibited solutions (added to prevent polymeri zation) usually contain 6 12% methyl alcohol. Formaldehyde is used in the manufacture of plastics and resins by reaction with phenols,urea, and melamine. It is used as a preservative,a disinfectant, and as a chemical intermediate.
History
Formaldehyde is a by-product of combustion of organic compounds, metabolism, and other natural processes. Formaldehyde results from wood combustion and elevated atmospheric concentrations can result from forest fires, as well as from urban pollution sources such as transportation. Formaldehyde has been identified as a significant indoor air pollutant. Building materials such as particleboard, plywood, and paneling are major sources of formaldehyde because they incorporate formaldehyde resins as bonding adhesives. Other sources of formaldehyde in the home are carpets, upholstery, drapes, tobacco smoke, and indoor combustion products. Formaldehyde may be emitted from building materials for several years after installation. In the two decades of the 1960s and 1970s, a half million homes in the United States used urea formaldehyde foam insulation, but health complaints led to its elimination as an insulator in the early 1980s. People react differently to formaldehyde exposure, but it is estimated that between 10% and 20% of the population will experience some reaction at concentrations as low as 0.2 parts per million. Formaldehyde irritates the eyes, nose, and throats, producing coughing, sneezing, runny nose, and burning eyes. More severe reactions result in insomnia, headaches, rashes, and breathing difficulties. Some states have established indoor air quality standards ranging from 0.05 to 0.5 ppm.
Uses
Formaldehyde is used in the manufactureof phenolic resins, cellulose esters, artificialsilk, dyes, explosives, and organic chemicals.Other uses are as a germicide, fungicide, anddisinfectant; in tanning, adhesives, waterproofingfabrics, and for tonic and chromeprinting in photography; and for treating skindiseases in animals. In vitro neutralizationof scorpion venom toxicity by formaldehydehas been reported (Venkateswarlu et al.1988).Formaldehyde constitutes about 50% ofall aldehydes present in the air. It is oneof the toxic effluent gases emitted fromburning wood and synthetic polymeric substancessuch as polyethylene, nylon 6, andpolyurethane foams. Firefighters have a greaterrisk to its exposure. Incapacitation fromthe toxic effluent gases is reported to occurmore rapidly from the combustion of syntheticpolymers than from that of naturalcellulose materials.Formaldehyde is directly emitted into theair from vehicles. It is released in traceamounts from pressed wood products suchas particleboard and plywood paneling, fromold “sick” buildings, and from cotton andcotton–polyester fabrics with selected crosslinkfinishes. Formation of formaldehyde hasbeen observed in some frozen gadoid fishdue to enzymic decomposition of the additivetrimethylamine oxide (Rehbein 1985).Its concentration can build up during frozenstorage of fish (Leblanc and Leblanc 1988;Reece 1985). It occurs in the upper atmosphere,cloud, and fog; it also forms inphotochemical smog processes.
Uses
More than half of the commercial formaldehyde produced is used to manufacture phenolic,urea, and melamine formaldehyde resins. Polyacetyl resins use another 5–10% of formaldehyde,and approximately 80% of formaldehyde goes into the resins and plastics industry.Phenolic-formaldehyde resins were the first synthetic plastics to be produced. The first plasticwas called Bakelite.Formaldehyde has traditionally been used as a preservative in biology and medical laboratoriesand in embalming fluid. Embalming fluids typically contain 5–15% formaldehyde, a significant percentage of alcohol, and other additives to perform certain functions, for example,bleaches and coloring to preserve skin color. Formaldehyde has been used to preserve deadbodies since 1900 and has several qualities that make it the preferred preservative. Foremostamong these is its low cost, but it also has several biochemical advantages: it kills germs andmicroorganisms, destroys decomposition enzymes, retards decomposition of proteins, andhardens body tissues.
Uses
Formaldehyde is used as the preservative; disinfectant; antiseptic; in embalming solutions; in the manufacture of phenolic resins, artificial silk, cellulose esters, dyes, urea, thiourea, melamine res ins, organic chemicals, glass mirrors and explosives; used in improving fastness of dyes on fabrics; in tanning and preserving hides; in mordanting and waterproofing fabrics; as a germicide and fungicide for vegetables and other plants; in destroying flies and other insects; in preserving and coagulating rubber latex; prevent mildewand spelt in wheat and rot in oats; used to ren der casein, albumin, and gelatin insoluble; in chemical analysis; as a tissue fixative; as a component of particle board and plywood; in the manufacture of pentaerythritol, hexamethylenetetramine and lkbutanediol; used in ceiling and wall insulation; in res ins used to wrinkle-proof fabrics; in photography for hardening gelatin plates and papers, for toning gelatin-chloride papers and for chrome printing and developing; intermediate in drug manufacture; pesticide intermediate; in the production of urea, phenolic melamine and acetale resins; in textile products; as an astringent, disinfectant, and preservative in cosmetics, metal-working fluids, shampoos, etc.; antiperspirant in cosmetics; anticracking agent in dental plastics; in anhidrotics; chipboard production; in cleaning products, disinfectants and deodorizers, dry-cleaning materials, and glues; in mineral-wool production, paints and coatings, paper industry, phenolic resins and urea plastics; in adhesives and footwear, photographic paper and solutions, polishes, printing materials, tanning agents, wart remedies, embalming solutions, fertilizers, wood composites, and insulation.
Production Methods
The industrial preparation of formaldehyde has occurred since the late 1800s and involvesthe catalytic oxidation of methanol: 2CH3OH(g) + O2(g) → 2CH2O(g).the oxidationtakes place at temperatures between 400°C and 700°C in the presence of metal catalysts. Metalsinclude silver, copper, molybdenum, platinum, and alloys of these metals. Formaldehyde iscommonly used as an aqueous solution called formalin. Commercial formalin solutions varybetween 37% and 50% formaldehyde. When formalin is prepared, it must be heated anda methanol must be added to prevent polymerization; the final formalin solution containsbetween 5% and 15% alcohol.
Preparation
Formalin is adjusted to pH 8 and urea is added to give a urea to formaldehyde ratio of about 1 :2.5 molar. The resulting solution is boiled under reflux for 1 hour. Butanol (1.5-2.0 mole per mole of urea) is then added together with a little xylene. The latter forms, with butanol and water, a ternary azeotrope which on distillation yields a condensate separating into an upper organic layer and a lower aqueous layer. By discarding the lower layer and returning the upper layer to the reactor, water is progressively removed from the system. After a substantial proportion of the water has been removed, an acid catalyst (e.g. phosphoric acid or phthalic anhydride) is added and heating is continued. When the required degree of reaction is attained, the solution is neutralized and concentrated to the desired solids content.
Definition
Formalin: a colourless solution of methanal (formaldehyde) in waterwith methanol as a stabilizer; r.d.1.075–1.085. When kept at temperaturesbelow 25°C a white polymer ofmethanal separates out. It is used asa disinfectant and preservative forbiological specimens.
General Description
Formaldehyde solution commercially formaldehyde solution is an aqueous solution containing 37% formaldehyde and 8-10% methanol. Formaldehyde solution can be activated by adding the catalytic amount of lanthanide triflate. This activated formaldehyde solution was employed for the smooth hydroxymethylation reaction of silyl enol ethers. Formaldehyde solution reacts with silyl enol ethers to afford the corresponding hydroxymethylated adducts in high yields.
Air & Water Reactions
The solution gives up formaldehyde vapors readily. These vapors are flammable over a wide vapor-air concentration range. Water soluble.
Reactivity Profile
FORMALDEHYDE, SOLUTION, reacts violently with strong oxidizing agents (hydrogen peroxide, performic acid, perchloric acid in the presence of aniline, potassium permanganate, nitromethane). Reacts with bases (sodium hydroxide, potassium hydroxide, ammonia), and with nitrogen dioxide (explosive reaction around 180°C). Reacts with hydrochloric acid to form highly toxic bis(chloromethyl) ether. Polymerization reaction with phenol may develop sudden destructive pressure [Bretherick, 5th ed., 1995, p.168].
Hazard
Moderate fire risk. Explosive limits in air 7– 73%. Toxic by inhalation, strong irritant, a carcinogen. (Solution) Avoid breathing vapor and avoid skin contact. Confirmed carcinogen.
Health Hazard
Formaldehyde is moderately toxic by skin contact and inhalation. Exposure to formaldehyde gas can cause irritation of the eyes and respiratory tract, coughing, dry throat, tightening of the chest, headache, a sensation of pressure in the head, and palpitations of the heart. Exposure to 0.1 to 5 ppm causes irritation of the eyes, nose, and throat; above 10 ppm severe lacrimation occurs, burning in the nose and throat is experienced, and breathing becomes difficult. Acute exposure to concentrations above 25 ppm can cause serious injury, including fatal pulmonary edema. Formaldehyde has low acute toxicity via the oral route. Ingestion can cause irritation of the mouth, throat, and stomach, nausea, vomiting, convulsions, and coma. An oral dose of 30 to 100 mL of 37% formalin can be fatal in humans. Formalin solutions can cause severe eye burns and loss of vision. Eye contact may lead to delayed effects that are not appreciably eased by eye washing.Formaldehyde is regulated by OSHA as a carcinogen (Standard 1910.1048) and is listed in IARC Group 2A ("probable human carcinogen"). This substance is classified as a "select carcinogen" under the criteria of the OSHA Laboratory Standard. Prolonged or repeated exposure to formaldehyde can cause dermatitis and sensitization of the skin and respiratory tract. Following skin contact, a symptom free period may occur in sensitized individuals. Subsequent exposures can then lead to itching, redness, and the formation of blisters
Fire Hazard
Toxic vapors such as carbon dioxide and carbon monoxide are generated during combustion. Explosion hazard: when aqueous formaldehyde solutions are heated above their flash points, a potential for explosion hazard exists. High formaldehyde concentration or methanol content lowers flash point. Reacts with nitrogen oxides at about 180; the reaction becomes explosive. Also reacts violently with perchloric acid-aniline, performic acid, nitromethane, magnesium carbonate, and hydrogen peroxide. When heated, irritant formaldehyde gas evolved from solution. The main products of decomposition are carbon monoxide and hydrogen. Metals such as platinum, copper, chromia, and alumina also catalyze the formation of methanol, methylformate, formic acid, carbon dioxide, and methane. Reacts with peroxide, nitrogen oxide, and performic acid causing explosions. Can react with hydrogen chloride or other inorganic chlorides to form bis-chloromethylether (BCME), a known carcinogen. Very reactive, combines readily with many substances, 40% solution is powerful reducing agent. Incompatible with amines, azo compounds, dithiocarbamates, alkali and alkaline earth metals, nitrides, nitro compounds, unsaturated aliphatics and sulfides, organic peroxides, oxidizing agents, and reducing agents. Aqueous solutions are unstable. Commercial formaldehyde-alcohol solutions are stable. Gas is stable in absence of water. Avoid oxidizing and alkaline materials. Hazardous polymerization may occur. Compound will polymerize with active organic materials such as phenol. Will polymerize violently in the presence of caustics and nitrides; (amines) exothermic reaction, (Azo compound) exothermic reaction giving off nitrogen gas, (caustics) heat generation and violent polymerization, (dithiocarbamates) formation of flammable gases and toxic fumes, formation of carbon disulfide may result, (alkali and alkaline earth metals) heat generation and formation of a flammable hydrogen gas.
Flammability and Explosibility
Formaldehyde gas is extremely flammable; formalin solution is a combustible liquid (NFPA rating = 2 for 37% formaldehyde (15% methanol), NFPA rating = 4 for 37% formaldehyde (methanol free)). Toxic vapors may be given off in a fire. Carbon dioxide or dry chemical extinguishers should be used to fight formaldehyde fires.
Chemical Reactivity
Reactivity with Water No reaction; Reactivity with Common Materials: No reactions; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Agricultural Uses
Microbiocide, Fungicide, Bactericide; Soil sterilent: Registered for use in the U.S. Not approved for use in EU countries. Formaldehyde has found wide industrial usage as a fungicide, germicide and in disinfectants. It is used most often in an aqueous solution stabilized with methanol (formalin). It is also a pesticide intermediate.
Trade name
DYNOFORM?; FANNOFORM?; FORMALITH?; FORMOL?; FYDE?; HERCULES 37 M6-8?; HOCH?; IVALON?; KARSAN?; LYSOFORM?; MAGNIFLOC 156C FLOCCULANT?; MORBICID?; STERIFORM?; SUPERLYSOFORM?
Contact allergens
Sources and uses of formaldehyde are numerous. Exposed people are mainly health workers, cleaners, painters, met alworkers, but also photographers (color developers) and carbonless copy paper users. Formaldehyde can induce contact urticaria. Formaldehyde may be the cause of sen sitization to formaldehyde releasers: benzylhemiformal, bromonitrodioxane, bromonitropropanediol (?), chloroal lylhexaminium chloride or Quaternium-15, diazolidinylu rea, dimethylol urea, dimethyloldimethylhydantoin or DMDM hydantoin, hexamethylenetetramine or methe namine, imidazolidinylurea, monomethyloldimethylhy dantoin or MDM hydantoin, N-methylolchloracetamide, paraformaldehyde and trihydroxyethylhexahydrotriazine or Grotan BK. Formaldehyde is used for the synthesis of many resins. Some of them, such as formaldehyde-urea and melamine formaldehyde resins, can be used in textiles and second arily release free formaldehyde (see Chap. 40). Other resins, such as p-tert-butylphenol formalde hyde resin or tosylamine formaldehyde resin, do not release formaldehyde.
Biochem/physiol Actions
Formaldehyde is the simplest aldehyde that denatures the bihelical regions of RNA and converts the polynucleotides into random coils. It is a genotoxic substance that significantly induces DNA-protein crosslinks (DPC), sister-chromatid exchanges, micronuclei formation and leads to cytotoxicity. It also induces tumors in the nasal epithelium of rats and supposed to be a human carcinogen.
Safety Profile
Confirmed carcinogen with experimental carcinogenic, tumorigenic, and teratogenic data. Human poison by ingestion. Experimental poison by ingestion, skin contact, inhalation, intravenous, intraperitoneal, and subcutaneous routes. Human systemic effects by inhalation: lachqmation, olfactory changes, aggression, and pulmonary changes. Experimental reproductive effects. Human mutation data reported. A human skin and eye irritant. If swallowed it causes violent vomiting and darrhea that can lead to collapse. Frequent or prolonged exposure can cause hypersensitivity leading to contact dermatitis, possibly of an eczematoid nature. An air concentration of 20 ppm is quickly irritating to eyes. A common air contaminant. Flammable liquid when exposed to heat or flame; can react vigorously with oxidizers. A moderate explosion hazard when exposed to heat or flame. The gas is a more dangerous fire hazard than the vapor. Should formaldehyde be involved in a fire, irritating gaseous formaldehyde may be evolved. When aqueous formaldehyde solutions are heated above their flash points, a potential for an explosion hazard exists. High formaldehyde concentration or methanol content lowers the flash point. Reacts with sodum hydroxide to yield formic acid and hydrogen. Reacts with NOx at about 180'; the reaction becomes explosive. Also reacts violently with perchloric acid + anhe, performic acid, nitromethane, magnesium carbonate, H2O2. Moderately dangerous because of irritating vapor that may exist in toxic concentrations locally if storage tank is ruptured. To fight fire, stop flow of gas (for pure form); alcohol foam for 37% methanol-free form. When heated to decomposition it emits acrid smoke and fumes. See also ALDEHYDES.
Potential Exposure
Formaldehyde has found wide indus trial usage as a fungicide, germicide; and in disinfectants and embalming fluids. It is also used in the manufacture of artificial silk and textiles, latex, phenol, urea, thiourea and melamine resins; dyes, and inks; cellulose esters and other organic molecules; mirrors, and explosives. It is also used in the paper, photographic, and furniture industries. It is an intermediate in drug manufacture and is a pesticide intermediate.
Carcinogenicity
Formaldehyde is known to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in humans and supporting data on mechanisms of carcinogenesis. Formaldehyde was first listed in the Second Annual Report on Carcinogens in 1981 as reasonably anticipated to be a human carcinogen based on sufficient evidence from studies in experimental animals. Since that time, additional cancer studies in humans have been published, and the listing status was changed to known to be a human carcinogen in the Twelfth Report on Carcinogens (2011).
Source
Formaldehyde naturally occurs in jimsonweed, pears, black currant, horsemint, sago cycas seeds (1,640 to 2,200 ppm), oats, beets, and wild bergamot (Duke, 1992). Formaldehyde was formed when acetaldehyde in the presence of oxygen was subjected to continuous irradiation (λ >2200 ?) at room temperature (Johnston and Heicklen, 1964). Schauer et al. (2001) measured organic compound emission rates for volatile organic compounds, gas-phase semi-volatile organic compounds, and particle phase organic compounds from the residential (fireplace) combustion of pine, oak, and eucalyptus. The gas-phase emission rates of formaldehyde were 1,165 mg/kg of pine burned, 759 mg/kg of oak burned, and 599 mg/kg of eucalyptus burned. Gas-phase tailpipe emission rates from California Phase II reformulated gasoline-powered automobiles with and without catalytic converters were 8.69 and 884 mg/km, respectively (Schauer et al., 2002).
Environmental Fate
Biological. Biodegradation products reported include formic acid and ethanol, each of which can further degrade to carbon dioxide (Verschueren, 1983).Photolytic. Major products reported from the photooxidation of formaldehyde with nitrogen oxides are carbon monoxide, carbon dioxide and hydrogen peroxide (Altshuller, 1983). In synthetic air, photolysis of formaldehyde gave hydrochloric acid andIrradiation of gaseous formaldehyde containing an excess of nitrogen dioxide over chlorine yielded ozone, carbon monoxide, nitrogen pentoxide, nitryl chloride, nitric acid and hydrochloric acid. Peroxynitric acid was the major photolysis product when chloChemical/Physical. Oxidizes in air to formic acid (Hartley and Kidd, 1987). Trioxymethylene may precipitate under cold temperatures (Sax, 1984). Polymerizes easily (Windholz et al., 1983). Anticipated products from the reaction of formaldehyde with ozone orhydroxyl radicals in air are carbon monoxide and carbon dioxide (Cupitt, 1980). Major products reported from the photooxidation of formaldehyde with nitrogen oxides are carbon monoxide, carbon dioxide and hydrogen peroxide (Altshuller, 1983).Reacts with hydrochloric acid in moist air forming bis(chloromethyl)ether. This compound may also form from an acidic solution containing chloride ion and formaldehyde (Frankel et al., 1974). In an aqueous solution at 25°C, nearly all the formaldehyde add
storage
work with formaldehyde should be conducted in a fume hood to prevent exposure by inhalation, and splash goggles and impermeable gloves should be worn at all times to prevent eye and skin contact. Formaldehyde should be used only in areas free of ignition sources. Containers of formaldehyde should be stored in secondary containers in areas separate from oxidizers and bases.
Shipping
UN1198 Formaldehyde solutions, flammable, Hazard Class: 3; Labels: 3-Flammable liquid, 8-Corrosive material. Cylinders must be transported in a secure upright position, in a well-ventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written permission of the owner. UN2209 Formaldehyde solutions, with not<25% formal dehyde, Hazard class: 8; Labels: 8-Corrosive material. UN3077 For solids containing varying amounts of formal dehyde : UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Purification Methods
It commonly contains added MeOH. Add KOH solution (1 mole KOH: 100 moles HCHO) to ~37% by weight aqueous formaldehyde solution (formalin), or evaporate to dryness, to give paraformaldehyde polymer which, after washing with water, is dried in a vacuum desiccator over P2O5 or H2SO4. Formaldehyde is regenerated by heating the paraformaldehyde to 120o under vacuum, or by decomposing it with barium peroxide. The monomer, a colourless flammable gas, is passed through a glass-wool filter cooled to -48o in a CaCl2/ice mixture to remove particles of polymer, then dried by passage over P2O5 and either condensed in a bulb immersed in liquid nitrogen or absorbed in ice-cold conductivity water. The gas or aqueous solutions have pungent suffocating odours, are LACHRYMATORY and suspected carcinogens, handle carefully. Formalin is a disinfectant and a preservative of dead animal and plant tissues. [Beilstein 1 IV 3017.]
Toxicity evaluation
The carbonyl atom is the electrophilic site of formaldehyde, making it react easily with nucleophilic sites on cell membranes and in body fluids and tissues such as the amino groups in protein and DNA. Higher concentrations of formaldehyde precipitate protein. It is probable that formaldehyde toxicity occurs when intracellular levels saturate formaldehyde dehydrogenase activity, allowing the unmetabolized intact molecule to exert its effects locally. Formaldehyde is a very strong crosslinking agent even in the low concentration range. The reaction mechanism of this agent is the initial addition of formaldehyde to a primary amine on either an amino acid residue or DNA base to yield a hydroxymethyl intermediate. Then the hydroxymethyl group condenses with a second primary amine to yield a methylene bridge.
Incompatibilities
Pure formaldehyde may polymerize unless properly inhibited (usually with methanol). May form explosive mixture with air. Incompatible with strong acids; amines, strong oxidizers; alkaline materials; nitrogen dioxide; performic acid; phenols, urea. Reaction with hydrochloric acid forms bis-chloromethyl ether, a carcino gen. Formalin is incompatible with strong oxidizers, alkalis, acids, phenols, urea, oxides, isocyanates, caustics, anhydrides.
Waste Disposal
Return refillable compressed gas cylinders to supplier. Incineration in solution of combus tible solvent. Consult with environmental regulatory agen cies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, trans portation, treatment, and waste disposal.
InChI:InChI=1/CH2O/c1-2/h1H2
Methanol and formaldehyde were formed as major products at a moderate conversion level (16percent) in the partial oxidation of methane by nitrous oxide in the presence of water over molybdenum oxide supported on silica.
The reactions 2CH3O2 -> 2CH3O + O2 (1a), 2CH3O2 -> CH3OH + HCHO + O2 (1b), and 2CH3O2 -> CH3COOH + O2 (1c) have been studied at temperatures between 248 and 573 K.At temperature above 373 K, the resulting decay traces were distorted away from pure second order at short wavelenghts (around 210 nm), owing to the presence of the hydroperoxy radicals formed via the nonterminating pathway (1a) and the subsequent rapid step CH3O + O2 -> HCHO + HO2 (2).This distortion enabled the nonterminating/terminating branching ratio, β, to be determined.Combining the present resultswith previously published work on the branching ratios gave lnβ=3.80-1470/T.Thus, although reaction 1 acts as a termination reaction under atmospheric conditions, it largely serves to convert CH3O2 into HO2 under combustion conditions.The temperature dependence of β enabled the real constant for the reaction k1, to be obtained over the entire experimental temperature range, giving k1 = 1.3E-13exp(365/T)cm31/molecule1/s, with ?2A/cm6molecule-2s-2 = 2.00E-28, ?2E/R/K2 = 1712, and ?2AE/R/cm3molecule-1s-1 = -5.61E-13.Absolute uncertainties, including contributions from both the experimental measurements and the dependence of k1 on various analysis parameters, are estimated to be 22percent, independent of temperature.No dependence of either the branching ratio or k1 on the total pressure was found.The mechanism of the title reaction is discussed and the present results are compared with existing studies of alkylperoxy self-reactions.The implications for combustion and atmospheric modeling are also discussed.
-
The recent discovery that the formaldehyde conjugates of doxorubicin and daunorubicin, Doxoform and Daunoform, are cytotoxic to resistant human breast cancer cells prompted the search for hydrolytically more stable anthracycline-formaldehyde conjugates. Doxoform and Daunoform consist of two molecules of the parent drug bound together with three methylene groups, two forming oxazolidine rings and one binding the oxazolidines together at their 3'amino nitrogens. The 4'-epimer of doxorubicin, epidoxorubicin, reacts with formaldehyde at its amino alcohol functionality to produce a conjugate, Epidoxoform, in 59% yield whose structure consists of two molecules of epidoxorubicin bound together with three methylene groups in a 1,6-diaza- 4,9-dioxabicyclo[4.4.1]undecane ring system. The structure was established from spectroscopic data and is consistent with products from reaction of simpler vicinal trans-amino alcohols with formaldehyde. Epidoxoforrn hydrolyzes at pH 7.3 to an equilibrium mixture with dimeric and monomeric epidoxorubicin-formaldehyde conjugates without release of formaldehyde or epidoxorubicin. The hydrolysis follows the rate law (A mutually implies B) mutually implies C + D where A (Epidoxoform) is in rapid equilibrium with B, and B is in slow equilibrium with C and D. The forward rate constant for A/B going to C+D gives a half-life of approximately 2 h at 37 °C. At equilibrium the mixture is stable for at least 2 days. At pH 6.0, hydrolysis proceeds with first-order kinetics to epidoxorubicin and formaldehyde with a half- life of 15 min at 37 °C. Epidoxoform and epidoxorubicin plus formaldehyde react with the self-complementary DNA octamer (GC)4 to yield five drug-DNA adducts which have structures analogous to the doxorubicin-DNA adducts from reaction of Doxoform with (GC)4. Epidoxoform is 3-fold more toxic to MCF-7 human breast cancer cells and greater than 120-fold more toxic to MCF-7/ADR resistant cells than epidoxorubicin. Epidoxoform in equilibrium with its hydrolysis products is greater than 25-fold more toxic to resistant cells with respect to epidoxorubicin.
-
Nucleophilic addition of the peroxynitrite anion, ONOO-, to the two prototypical carbonyl compounds, acetaldehyde and acetone, was investigated in the pH interval 7.4-14. The process is initiated by fast equilibration between the reactants and the corresponding tetrahedral adduct anion, the equilibrium being strongly shifted to the reactant side. The adduct anion also undergoes fast protonation by water and added buffers. Consequently, the rate of the bimolecular reaction between ONOO- and the carbonyl is strongly dependent on the pH and on the concentration of the buffer. The pKa of the carbonyl-ONOO adduct was estimated to be 11.8 and 12.3 for acetone and acetaldehyde, respectively. It is shown that both the anionic and the neutral adducts suffer fast homolysis along the weak O-O bond to yield free alkoxyl and nitrogen dioxide radicals. The yield of free radicals was determined to be about 15% with both carbonyl compounds at low and high pH, while the remainder collapses to molecular products in the solvent cage. The rate constants for the homolysis of the adducts vary from ca. 3 x 105 to ca. 5 x 106 s-1, suggesting that they cannot act as oxidants in biological systems. This small variation around a mean value of about 106 s-1 suggests that the O-O bond in the adduct is rather insensitive to its protonation state and to the nature of its carbonyl precursor. An overall reaction scheme was proposed, and all the corresponding rate constants were evaluated. Finally, thermokinetic considerations were employed to argue that the formation of dioxirane as an intermediate in the reaction of ONOO- with acetone is an unlikely process.
Rate coefficients for the gas-phase reactions of Cl atoms with β-ocimene and camphene were determined to be (in units of 10-10 cm3 per molecule per s) 5.5 ± 0.7 and 3.3 ± 0.4, respectively. The experiments were performed by the relative technique in an environmental chamber with FTIR detection of the reactants at 298 K and 760 torr. Product identification experiments were carried out by gas chromatography with mass spectrometry detection (GC-MS) using the solid-phase microextraction (SPME) method employing on-fiber carbonyl compound derivatization with o-(2,3,4,5,6-pentafluorobenzyl) hydroxylamine hydrochloride. An analysis of the available rates of addition of Cl atoms and OH radicals to the double bond of alkenes and cyclic and acyclic terpenes with a conjugated double bond at 298 K is presented. The atmospheric persistence of these compounds was calculated taking into account the measured rate coefficients. In addition, tropospheric chemical mechanisms for the title reactions are postulated.
-
-
-
-
-
The reactions of atomic oxygen with CH2Cl2 and CH2Br2 trapped in argon matrices have been studied by FTIR spectroscopy.O(1D) and O(3P) were generated in situ by UV photolysis of co-deposited ozone.Products were identified by employing 18O and scrambled 18O/16O ozone as well as deuterated methylene halides.Kinetic studies performed on both CH2Cl2 and CH2Br2 with O(1D) under the same experimental conditions allowed the reaction pathway to be determined.With CH2Br2 as parent molecule, three routes were evident leading to (i) CHOBr,(ii) CO...(HBr)2, and (iii) CH2O.With CH2Cl2 as parent molecule, only the first two channels were observed.Carbonyl compounds rapidly decomposed under irradiation, and CO...(HX)2 was also produced as a secondary species.
-
-
-
We report here on the multiproton-multielectron electrochemical reduction of CO2 in homogeneous solution by using (TOA)6[α-SiW11O39Co(-)] (TOA = tetraoctyl ammonium; - = vacant position in the coordination sphere of Co) as an electrocatalyst. First, the electrochemical behavior of (TOA)6[α-SiW11O39Co(-)] was analyzed in detail by cyclic voltammetry in dichloromethane, studying the influence of the presence of protons and/or CO2. These preliminary results were further used to optimize the conditions of electrolysis in terms of reduction potentials. Analysis of the electrolysis products in the gas and liquid phases show the formation of CO and HCHO without formation of H2. Our results tend to show that the (TOA)6[α-SiW11O39Co(-)] polyoxometalate is a catalyst for CO2 electroreduction, with unique selectivity. The cobalt derivative of the silico-undecatungstate [α-SiW11O39Co(-)]6- is a catalyst for the multiproton-multielectron electrochemical reduction of CO2, with unique selectivity.
A fluorometric/colorimetric dual-channel chemosensor based on a hydrazine-substituted BODIPY probe has been successfully fabricated for the detection of RDX and PA. The chemosensor displays turn-on fluorescence behavior upon RDX with a detection limit of 85.8 nM, while showing a turn-off response to PA with a detection limit of 0.44 μM. Meanwhile, an obvious color difference is observed by the naked-eye after the reaction for RDX. Thus, in application, a two-to-two logic gate is constructed for potential application in explosives detection. Additionally, portable equipment is also developed for in situ determination of RDX.
The kinetics of the reaction of CH3O with H have been studied under pseudo-first-order conditions with an excess of H using an isothermal discharge-flow reactor.Three different CH3O sources were used and the decay of was monitored by laser-induced fluorescence (LIF) as a function of .A second-order rate coefficient of (2.0 +/- 0.6) x 1013 cm3 mol-1 s-1 was determined for reaction CH3O + H -> products at room temperature and a slight positive temperature dependence was observed between 298 and 490 K.Formaldehyde formation was found to be the dominant reaction path (81 +/- 12percent).Further identified products were OH (7 +/- 3percent) and methanol (a few percent) which were produced by the decomposition and stabilization, respectively, of the initially formed bound adduct.
-
A new analogue of gambierone, 44-methylgambierone, was isolated from the benthic dinoflagellate Gambierdiscus australes collected from Raoul Island (Rangitahua/Kermadec Islands). This molecule has been previously reported as maitotoxin-3. The structure of 44-methylgambierone was elucidated using 1D- and 2D-nuclear magnetic resonance spectroscopy and mass spectrometry techniques. The nine-ring polyether backbone (A–I) and functional groups (carbonyl, terminal diol, 1,3-diene and monosulphate) are the same for both compounds with the addition of an olefinic methyl group being the only modification in 44-methylgambierone.
Intermolecular, intramolecular, and intrasubstituent kinetic deuterium isotope effect studies have been carried out on secondary amine monooxygenase, the only heme-containing monooxygenase other than cytochrome P-450. Secondary amine monooxygenase exhibits an active site structure similar to myoglobin but has a reactivity, oxidative N-dealkylation, in common with those demonstrated by P-450. An mfermolecular isotope effect was not observed for N-dealkylation of dimethylamine, indicating that C-H bond cleavage is not the rate limiting step. The intramolecular isotope effect is also masked by a high commitment to catalysis and/or the absence of substrate methyl group interchange at the active site. A net intrinsic kinetic deuterium isotope effect of 1.76 has been revealed via an intrasubstituent isotope effect study of competition between D and H atoms within each methyl group of dimethylamine in 1,1,1′,1′-tetradeuteriodimethylamine. This value is essentially identical to that previously reported for P-450 and is much smaller than values observed for other histidine-ligated heme proteins such as myoglobin and the peroxidases (Miwa, G. T. et al. J. Biol. Chem. 1983, 258, 14445-14449). The small isotope effect is most consistent with a deprotonation mechanism in which significant H-atom tunneling is absent, rather than a H-atom abstraction mechanism. It has been shown that the identity of the eventual proton acceptor is important in determining the magnitude of the intrinsic isotope effect for deprotonations (Dinnocenzo, J. P.; Banach, T. E. J. Am. Chem. Soc. 1989, 111, 8646-8653). Therefore, a relatively nonpolar proton acceptor environment within secondary amine monooxygenase, like that of cytochrome P-450, is implicated by close agreement in the isotope effects of these two proteins. Since cytochrome P-450 has a thiolate proximal ligand and secondary amine monooxygenase has a histidine proximal ligand, the identity of the heme proximal ligand appears to be less important than the nature of the distal active site environment in these N-dealkylation reactions. Thus, secondary amine monooxygenase resembles myoglobin and the peroxidases in its heme ligation and spectroscopic properties but catalyzes N-dealkylations via a mechanism that is similar to that employed by the other heme-containing monooxygenase, cytochrome P-450.
Multienzymatic cascade reactions have garnered the attention of many researchers as an approach for converting CO2 into methanol. The cascade reaction used in this study includes the following enzymes: a formate dehydrogenase (ClFDH), a formaldehyde dehydrogenase (BmFaldDH), and an alcohol dehydrogenase (YADH) from Clostridium ljungdahlii, Burkholderia multivorans, and Saccharomyces cerevisiae, respectively. Because this cascade reaction requires NADH as a cofactor, phosphite dehydrogenase (PTDH) was employed to regenerate the cofactor. The multienzymatic cascade reaction, along with PTDH, yielded 3.28 mM methanol. The key to the success of this cascade reaction was a novel formaldehyde dehydrogenase, BmFaldDH, the enzyme catalyzing the reduction of formate to formaldehyde. The methanol yield was further improved by incorporation of 1-ethyl-3-methylimidazolium acetate (EMIM-Ac), resulting in 7.86 mM of methanol. A 500-fold increase in total turnover number was observed for the ClFDH-BmFaldDH-YADH cascade system compared to the Candida boidinii FDH-Pseudomonas putida FaldDH-YADH system. We provided detailed insights into the enzymatic reduction of CO2 by determining the thermodynamic parameters (Kd and ΔG) using isothermal titration calorimetry. Furthermore, we demonstrated a novel time-dependent formaldehyde production from CO2. Our results will aid in the understanding and development of a robust multienzyme catalyzed cascade reaction for the reduction of CO2 to value-added chemicals.
Several phenothiazine derivatives have been shown to cause reproductive toxicity. The biochemical mechanisms responsible for these effects are not fully understood at present. In this study, we investigated hydrogen peroxide-dependent oxidation of six phenothiazines by purified lipoxygenase from soybean (SLO) and human term placenta (HTPLO). Chlorpromazine was employed as the prototype phenothiazine drag. Chlorpromazine was easily demethylated releasing formaldehyde when incubated at pH 7.0 and 6.5 with SLO or HTPLO, respectively, in the presence of hydrogen peroxide. The reaction was linear with respect to time, exhibited dependence on the amount of enzyme, and the concentration of chlorpromazine and hydrogen peroxide. Under the optimal assay conditions, the estimated Vmax values for chlorpromazine N- demethylation were 139 and 7.2 nmoles/min/mg of SLO and HTPLO, respectively. Collectively, the results suggest an enzymatic nature of the reaction. In the presence of gossypol and NDGA, the classical inhibitors of different lipoxygenases, the formaldehyde production was significantly decreased, as expected. Similar to SLO, the generation of chlorpromazine cation radical, an initial oxidation product with an absorption maximum at 525 nm, was also observed with HTPLO. The radical generation was detectable only under acidic conditions (pH 3.5-4.5). The formaldehyde production was also decreased by BHT and BHA, suggesting a radical nature of the SLO-mediated chlorpromazine N-demethylation. Reduced glutathione, ascorbate, and dithiothreitol suppressed the rate of SLO-dependent formaldehyde generation, presumably due to the reduction of the cation radical back to chlorpromazine in a concentration-dependent manner. Besides chlorpromazine, SLO also oxidized promazine, triflupromazine, trifluperazine, trimeprazine, and perphenazine, albeit at different rates, in the presence of hydrogen peroxide. The evidence gathered in this in vitro study suggests that phenothiazines can undergo peroxidative N-demethylation via lipoxygenase pathway. The role of this biochemical mechanism in the in vivo developmental toxicity of phenothiazines remains to be established.
-
An attempt to carry out a rigorous thermodynamic treatment of the question of the limiting efficiency of chemiluminescence is described. The reactions requiring additional activation and the activationless chemiluminescent reactions are treated separately.
-
The laser thermal lens analysis of formaldehyde is reported. Two systems were tested, the first using a single laser in combination with a single diode detector and the second using two lasers in conjunction with a photodiode array detector. The formaldehyde solutions were prepared for colorimetry using the NIOSH method based on chromotropic acid. Improved sensitivity over standard absorption techniques is observed with enhancement factors up to 20.0 based on detection of absorptivity as low as 9 x 10-5 cm-5 which corresponds to a concentration of 1.5 x 10-8 M. With collection efficiency of 95% for sampling solutions, this supports facile detection of formaldehyde in the parts-per-billion region in air. The laser thermal lens analysis of formaldehyde is reported. Two systems were tested, the first using a single laser in combination with a single diode detector and the second using two lasers in conjunction with a photodiode array detector. The formaldehyde solutions were prepared for colorimetry using the NIOSH method based on chromotropic acid. Improved sensitivity over standard absorption techniques is observed with enhancement factors up to 20. 0 based on detection of absorptivity as low as 9 multiplied by 10** minus **5 cm** minus **1 which corresponds to a concentration of 1. 5 multiplied by 10** minus **8 M. With collection efficiency of 95% for sampling solutions, this supports facile detection of formaldehyde in the parts-per-billion region in air.
Methanol oxidation kinetics were measured on Pt wires in a flow reactor at pressures between 30 and 130 Pa.The kinetics were measured as a function of oxygen-to-methanol equialence ratio Φ and wire temperature.In methanol-lean feeds (Φ 1) CO, H2, H2CO, CO2, and H2O were observed.Experiments with 18O2 showed that the principal mathanol oxidation pathway does not involve C-O bond dissociation.However, the 18O2 experiments, together with other features of the methanol oxidation data, also provided evidence for a minor oxidation pathway (accounting for less than 1 percent of the product CO2) which proceeds through a carbon intermediate.Interpretation of the CH3OH oxidation data was aided by comparison with data obtained previously for H2CO and CO oxidation (McCabe, R.W.; McCready, D.F.Chem.Phys.Lett. 1984. 111. 89).In particular, similarities between CH3OH oxidation and H2CO and CO oxidation indicate that the principal oxidation pathways for CH3OH, H2CO, and CO are kinetically homologous , i.e. they share the common feature of an adsorbed CO intermediate.A mathematical model is presented which describes the principal CH3OH oxidation pathway as a series reaction involving adsorbed H2CO and CO intermediates.Formaldehyde may not be the only adsorbed H2CO intermediate.Consistent with experimental results, the model predicts that inhibition by adsorbed CO should be weaker for CH3OH and H2CO oxidation than for CO oxidation.The model leads to a simplified, generalized expression for the rates of CH3OH, H2CO, and CO oxidation to CO2, thus reflecting the homologous nature of these reactions.The main effect of the minor pathway is to decrease the rate of the major pathway at Φ > 0.1.Additionally, the minor pathway accounts for certain features of methanol oxidation, including rate instabilities at certain values of Φ and deactivation of Pt wires in pure methanol, observed both in the present study and in preious studies in other laboratories.
The quenching of the excited state of the uranyl ion by aliphatic alcohols has been studied under conditions where the luminescence decay is bi-exponential.In agreement with previous reports, the quenching is strongly dependent on the type of alcohol, and shows a marked deuterium isotope effect.This is consistent with the major process for quenching being α-hydrogen-atom abstraction by (UO22+)*.Support for this comes from agreement between the isotope effects observed for quenching and for product formation.In an earlier study, the bi-exponential decay was interpreted in terms of a reversible crossing between two states, U* and X*.Analysis of the quenching data indicates that these have very similar reactivity towards alcohols.Results are also given for the decay of the intermediate hydroxyalkyl radicals in the presence of uranyl ion, and suggest that formation of the final organic product normally involves metal-ion oxidation.
A huge variety of silver based ternary sulfide semiconductors (SCs) have been considered for the sustainable advancement of renewable energy sources. Herein, we have synthesized two important classes of newly emerging semiconductor nanocrystals (NCs) Ag3SbS3 (SAS), i.e. hexagonal and monoclinic by simply tuning the solvent polarity, of which the second one has been synthesized in a phase pure NC for the first time by the thermal decomposition of silver and antimony based dithiocarbamate (~N-CS2-M) complexes. Interestingly, these two systems exhibit two different semiconducting (SC) properties and band gaps; hexagonal SAS has a p type (Eg ~ 1.65 eV) whereas monoclinic SAS has an n type (Eg ~ 2.1 eV) character. For the first time ever we have designed a reducing working electrode (i.e. cathode) by modifying the rotating disc electrode (RDE) with hexagonal SAS that exhibits excellent electrochemical oxygen reduction reaction (ORR) activity (Eonset = 1.09 V vs. RHE and average number of electron transfer: 3.89) comparable to that of the highly expensive Pt/C (Eonset = 0.88 V vs. RHE and average number of electron transfer: 3.92). Density functional theory (DFT) investigation confirms the corroborations of experimental data with theoretical implications. In addition, the electrode fabricated from monoclinic SAS acts as an efficient photoanode which exhibits higher photoelectrochemical (PEC) methanol oxidation reaction (MOR) activity under illumination in alkaline medium compared to that of standard TiO2 grown on an indium tin oxide (ITO) coated glass slide. On illumination, the relative photocurrent density at the onset potential has been obtained to be 845 which is a very significant experimental output with respect to any other TiO2 or Pt?TiO2 based photocatalysts for this application. The physicochemical stability and reusability of both materials were supported by 50 hours of extended electrochemical chronoamperometric measurements and powder XRD and the TEM analyses after electrocatalysis. This study explores a possible pathway for designing simple and less expensive but catalytically efficient silver based ternary sulfide NC systems for developing an SC material to reduce the energy crisis in the near future.
-
The synthesis of polyoxymethylene dimethyl ethers (DMMx) with a selectivity of 84.3 % by direct oxidation of dimethyl ether (DME) was realized over 30 %Ti(SO4)2/active carbon (AC) catalyst. This process also significantly promotes the growth of the C?O chain. The catalytic performances of Ti(SO4)2/AC and Ti(SO4)2/graphene (G) catalysts differ largely for DME oxidation reaction, although both AC and G are carbon materials. The carbonyl and hydroxyl groups on the surface of the carbon-based catalysts play an important role in DME direct oxidation to DMMx. Owing to the differences of surface structure and chemical properties of AC and G materials, the different interaction between the Ti(SO4)2 and supports remarkably affects the sulfate structures on the supports surface and leads to the large differences in the acid and redox properties of catalysts.
-
A transient ultraviolet adsorption attributed to the formyl radical, HCO, was produced by flash photolysis of water vapor in the presence of CO.For CO saturated with H2O at a total pressure of 1 atm, the formation of HCO is complete within c.a. 60 μs, after which it undergoes second-order decay, with k/ε(230 nm) = (1.5 +/- 0.2)e7 cm s-1.During the relatively slow formation reaction (H + CO (+M) -> HCO), the competing reaction H + HCO -> H2 + CO is significant.By employing the H2O + CH4 system as an actinometer to determine the amount of H2O dissociated by the flash (discussed elsewhere) and by computer modeling the observed formation and decay of HCO as a function of CO pressure, the following absolute rate constants were obtained: k3(H+CO(+M)->HCO) + (3.6 +/- 0.9)e7 M-2 s-1 for M = CO, (5.8 +/- 0.6)e7 M-2 s-1 for M = CH4, and (3.8 +/- 0.4)e7 M-2 s-1 for M = H2, k4(HCO+HCO->products) = (1.4 +/- 0.3)e10 M-2 s-1, and k5(H+HCO->products) = (6.9 +/- 1.7)e10 M-2 s-1.The molar extinction coefficient ε(HCO,230 nm) = 941 +/- 171 M-1 cm-1.
The complex Os(II)(bipy)2(CO3) undergoes a photoredox reaction in acetonitrile/H2O yielding [Os(VI)(bipy)2(O)2]2 + and formaldehyde. The reactive excited state is suggested to be of the (Os(II) to π?carbonate) MLCT type.
Smog chamber/FTIR techniques were used to determine rate constants of k(Cl + i-butanol) = (2.06 ± 0.40) × 10-10, k(Cl + i-butyraldehyde) = (1.37 ± 0.08) × 10-10, and k(OH + i-butanol) = (1.14 ± 0.17) × 10-11 cm3 molecule-1 s-1 in 700 Torr of N2/O2 diluent at 296 ± 2K. The UV irradiation of i-butanol/Cl 2/N2 mixtures gave i-butyraldehyde in a molar yield of 53 ± 3%. The chlorine atom initiated oxidation of i-butanol in the absence of NO gave i-butyraldehyde in a molar yield of 48 ± 3%. The chlorine atom initiated oxidation of i-butanol in the presence of NO gave (molar yields): i-butyraldehyde (46 ± 3%), acetone (35 ± 3%), and formaldehyde (49 ± 3%). The OH radical initiated oxidation of i-butanol in the presence of NO gave acetone in a yield of 61 ± 4%. The reaction of chlorine atoms with i-butanol proceeds 51 ± 5% via attack on the α-position to give an α-hydroxy alkyl radical that reacts with O2 to give i-butyraldehyde. The atmospheric fate of (CH3)2C(O)CH 2OH alkoxy radicals is decomposition to acetone and CH2OH radicals. The atmospheric fate of OCH2(CH3)CHCH 2OH alkoxy radicals is decomposition to formaldehyde and CH 3CHCH2OH radicals. The results are consistent with, and serve to validate, the mechanism that has been assumed in the estimation of the photochemical ozone creation potential of i-butanol.
The primary products and the rate of the reaction of methyl radicals with oxygen atoms in the gas phase at room temperature have been studied using three different experimental arrangements: (A) laser flash photolysis to produce CH3 and O from the precursors CH3I and SO2 (the educts and the products were detected by quantitative FTIR spectroscopy); (B) the coupling of a conventional discharge flow reactor via a molecular sampling system to a mass spectrometer with electron impact ionization, which allowed the determination of labile and stable species; (C) laser induced multiphoton ionization combined with a TOP mass spectrometer-molecular beam sampling-flow reactor, which was used for the specific and sensitive detection of the CH 3, CD3, C2H5 and C2D 5 radicals and the determination of rate coefficients. The branching ratio of the reaction channels was determined by the experimental arrangements (A) and (B) leading to CH3 + O → HCHO + H (55 ± 5)% → CO + H2 + H (45 ± 5)%. The rate coefficients of the normal and deuterated methyl and ethyl radicals with atomic oxygen showed no isotope effect: k(CD3 + O)k(CH3 + O) = 0.99 ± 0.12, k(C2D5 + O)k(C2H5 + O) = 1.01 ± 0.07 (statistical error, 95% confidence level). The absolute rate coefficient of the reaction CH3 + O was derived with reference to the reaction C2H5 + O (k = 1.04 × 1014 cm3 mol-1 s-1) leading to k(CH3 + O) = (7.6 ± 1.4) × 1013 cm3 mol-1 s-1. The Owner Societies 2005.
-
-
This study describes the potential advantages of task-specific functionalization of graphene for carbon dioxide capture and conversion. An ionic liquid was employed as a functional moiety on a graphene catalyst support since it shows reversible interactions with CO2 molecules. In this study, graphene was synthesized by a hydrogen-assisted low-temperature-exfoliation method and functionalized using a polymerized ionic liquid (PIL). Adsorption analysis shows that PIL functionalization improves the CO2 adsorption capacity of graphene significantly. Catalytic nanoparticles were dispersed over the catalyst supports by a microwave assisted polyol reduction method and the catalysts were employed as the cathode catalyst in a polymer electrolyte membrane (PEM) CO2 conversion cell. The cell hydrogenates CO2 into formic acid at the cathode, which was quantified by spectrophotometry. The PIL functionalized support shows a higher formic acid formation rate compared to a pure support under similar experimental conditions since PIL functionalization improves the interactions between the catalyst support and the CO2 molecules. The cells were tested with discontinuous and continuous CO2 supplies. This journal is
Oxidation kinetics of triethanolamine by ceric ammonium sulfate in aqueous sulfuric acid has been studied spectrophotometrically in contexts of many physicochemical processes. Stoichiometry of the reaction is found to be 1:6. Contrary to the literature findings the reaction proceeds without the presence of any transition metals acting as catalysts. Oxidation kinetics shows unit order dependence on oxidant, Ce(IV) and substrate, triethanolamine as well. Complex, fractional inverse order dependence on [H+], unaltered rate in the presence of added products at the initial stage and inverse dependence on added salt, sodium bisulfate are the findings. With increase in the solvent polarity, rate of the reaction also increased. Activation and thermodynamic parameters are computed from the temperature dependence observations. Suitable kinetic model and explanations are provided considering all the findings with a proposal of formation of an activated complex of the type [Ce(IV)-triethanolamine]. Graphical abstract: [Figure not available: see fulltext.]
The invention provides a method for preparing formaldehyde from ethylene glycol by photocatalytic oxidation. According to the method, ethylene glycol is taken as a substrate, air or oxygen is taken asan oxygen source, and a C-C bond cracked product, namely, formaldehyde can be generated under illumination in presence of a catalyst. The conditions are mild, the oxidation efficiency and the productyield are high, and the air or the oxygen is taken as the oxygen source under the illumination condition, so that the method is economical, environmentally friendly and green, meets the strategy of sustainable developed energy and has broad application prospect.
A series of molybdenum-incorporated mesoporous silica (Mo-KIT-6) catalysts were successfully synthesized by a one-pot hydrothermal synthesis method, and were applied in the selective oxidation of methane to formaldehyde using oxygen as an oxidizing agent under atmospheric pressure. Comparatively, the corresponding supported catalysts (Mo/KIT-6) were prepared by incipient-wetness-impregnation method. The results of the small angle XRD, nitrogen adsorption/desorption isotherms, UV-vis, H2-TPR and UV-Raman spectroscopy characterization combined with the catalytic activity tests demonstrated that molybdenum atoms were inserted into the framework of the mesoporous materials for the Mo-KIT-6 catalysts and the highly dispersed MoO bonds dominantly existed, which were responsible for the efficient selective formation of formaldehyde. However, for Mo/KIT-6 catalysts, the molybdenum oxide species were mainly loaded on the surface or inside the outer pore channels of the support and abundant emergence of the Mo-O-Mo bond played a major role in the activation of methane to COx. Furthermore, with equivalent molybdenum content, the methane selective oxidation performance of 8Mo-KIT-6 was obviously better than that of 4.6Mo/KIT-6, and the formaldehyde yield (2.1%) of 8Mo-KIT-6 was 2.3 times as much as that (0.9%) of 4.6Mo/KIT-6.In situandoperandoUV-Raman results demonstrated that the structures of the MoOxactive sites have a strong effect on the formation and elimination of carbon deposition during the separated redox reaction with methane and O2, respectively. The polymerized MoOxactive sites are favorable for the formation of graphitic carbon (G), which is called ordered carbon, while the isolated MoOxactive sites are favorable for the formation of disordered carbon (D). The reduced highly dispersed MoOxactive sites incorporated in the framework of silica are more easily reoxidized than those on the supported catalysts.
The effect of various iron phosphate and oxide catalysts on the direct oxidation of methane (CH4) to formaldehyde (HCHO) with molecular oxygen (O2) as the sole oxidant was studied using a fixed-bed flow reactor. Five crystalline iron-containing catalysts (FePO4, Fe3O3(PO4), Fe4(P2O7)3, Fe2P2O7, and α-Fe2O3) with different iron coordination geometries, iron oxidation states, and Fe/P ratios were synthesized by the sol-gel method using malic acid or aspartic acid. The Fe/P molar ratio had a significant effect on the oxidation catalysis; CH4 conversion increased with the Fe/P molar ratio, although the selectivity to HCHO decreased. Trigonal FePO4 nanoparticles synthesized by the malic acid-aided method with an Fe/P molar ratio of 1/1 exhibited the highest activity for the selective formation of HCHO among the catalysts tested, including FePO4 synthesized by a conventional method. Despite the much higher oxidizing ability of Fe2O3 than FePO4, the oxidation of CH4 using Fe2O3 resulted in the formation of only CO2. In contrast, the temperature-programmed reaction of FePO4 with CH4 gave Fe2P2O7 with the formation of HCHO as a primary product, and Fe2P2O7 reacted with O2 to regenerate FePO4. Based on mechanistic studies including the catalyst effect, kinetics, pulse-reaction experiments, and IR spectroscopy, the bulk structural change between FePO4 and Fe2P2O7 is not involved during the catalysis and the surface redox and acid-base properties of FePO4 are considered to play an important role in CH4 oxidation with the structure preservation of bulk FePO4. The combination of redox-active Lewis acidic iron sites and weakly-basic phosphate units likely contributes to the C-H activation of CH4 and the suppression of complete oxidation to CO2, respectively.
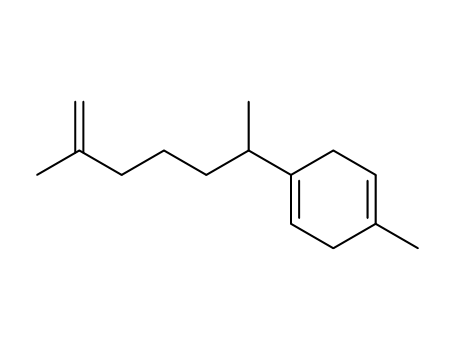
2-methyl-6-(4-methyl-cyclohexa-1,4-dienyl)-hept-1-ene

formaldehyd

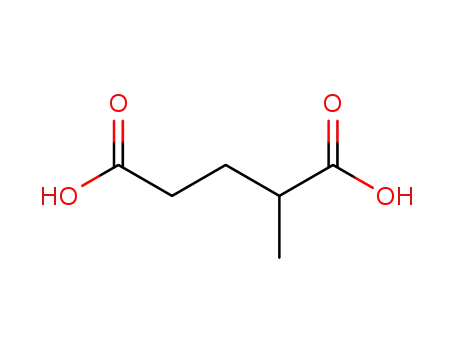
2-methylglutaric acid

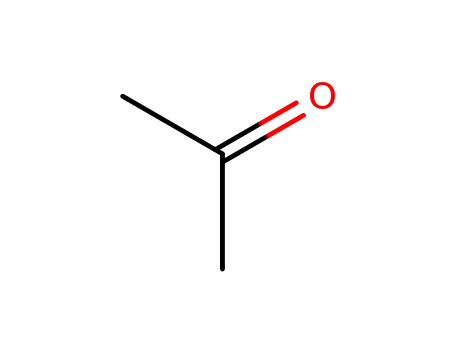
acetone
| Conditions | Yield |
|---|---|
|
Ozonolyse, anschliessende Oxydation mit NaOBr;
|
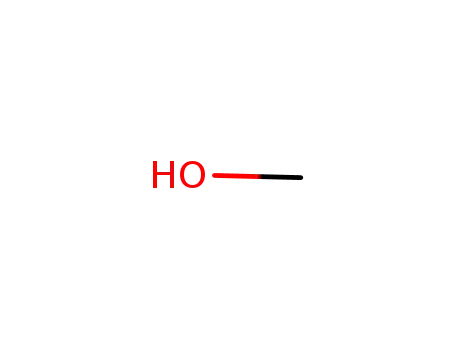
methanol

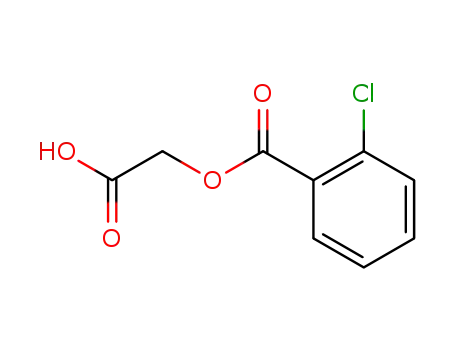
(2-Chlorbenzoyloxy)essigsaeure

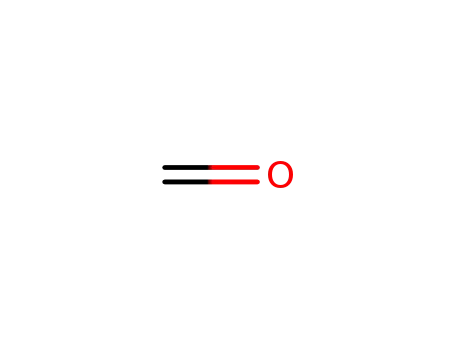
formaldehyd

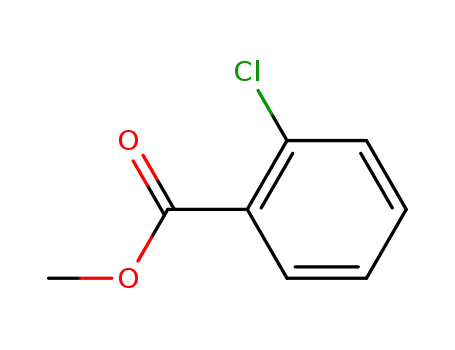
methyl chlorobenzoate

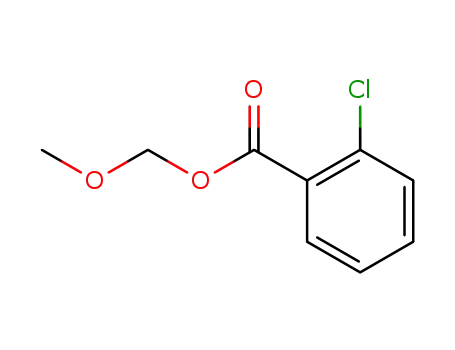
2-Chlorbenzoesaeure-methoxymethylester

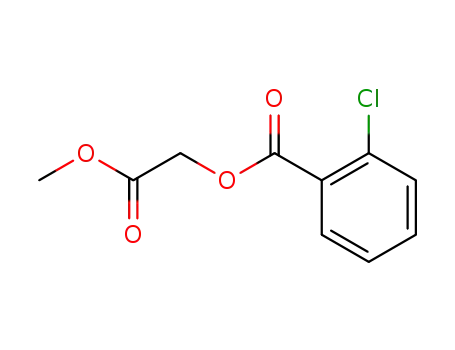
(2-Chlorbenzoyloxy)essigsaeure-methylester
| Conditions | Yield |
|---|---|
|
With
triethylamine;
at -20 ℃;
Product distribution;
electrolysis;
|
8 % Chromat. |
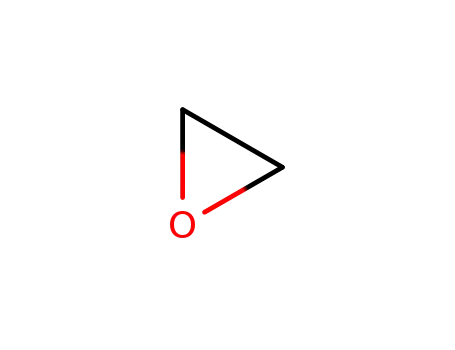
oxirane
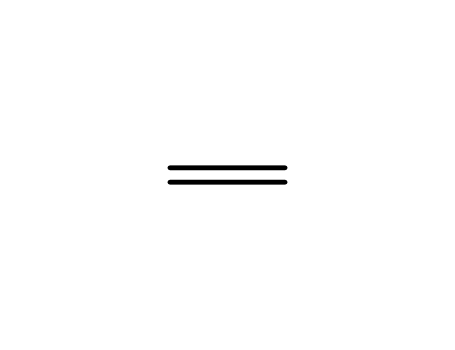
ethene
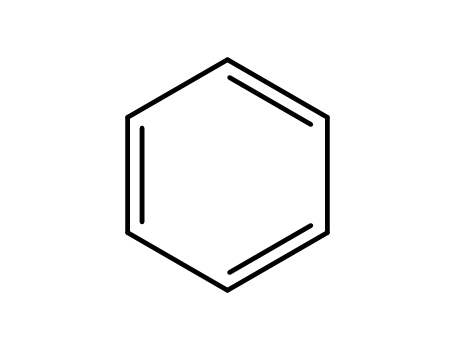
benzene

diazomethane
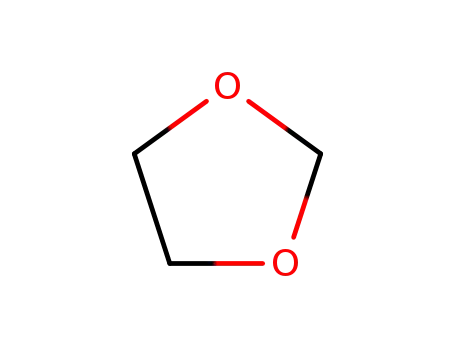
1,3-DIOXOLANE
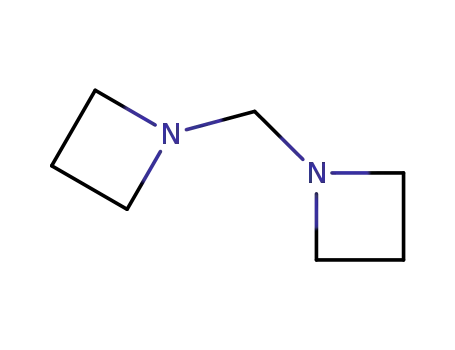
Methylen-bis-azetidin
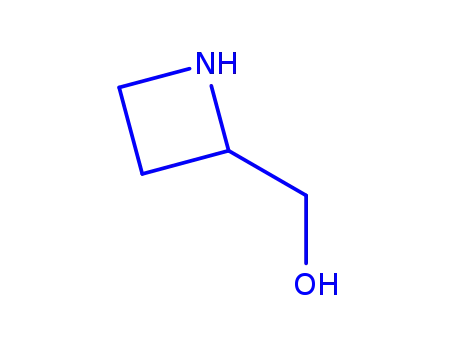
azetidin-2-ylmethanol
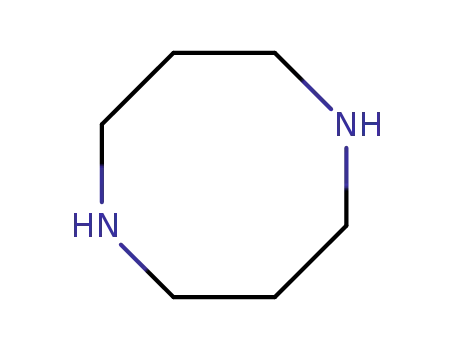
1,5-diazacyclooctane
CAS:300-57-2
CAS:104934-52-3
CAS:286438-45-7
CAS:99586-26-2