Your Location:Home >Products >OLED intermediates >Fluorenes >286438-45-7
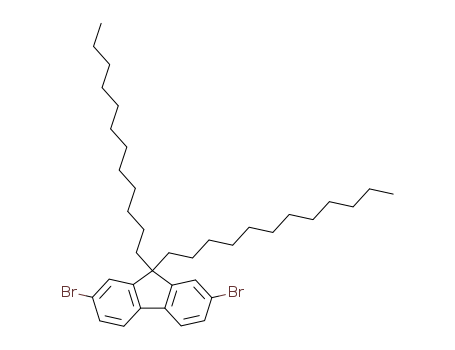

Product Details
Chemical Properties
light yellow powder
Uses
It is an intermediate for polymeric light-emitting diodes.
InChI:InChI=1/C37H56Br2/c1-3-5-7-9-11-13-15-17-19-21-27-37(28-22-20-18-16-14-12-10-8-6-4-2)35-29-31(38)23-25-33(35)34-26-24-32(39)30-36(34)37/h23-26,29-30H,3-22,27-28H2,1-2H3
The separation and isolation of semiconducting and metallic single-walled carbon nanotubes (SWNTs) on a large scale remains a barrier to many commercial applications. Selective extraction of semiconducting SWNTs by wrapping and dispersion with conjugated polymers has been demonstrated to be effective, but the structural parameters of conjugated polymers that dictate selectivity are poorly understood. Here, we report nanotube dispersions with a poly(fluorene-co-pyridine) copolymer and its cationic methylated derivative, and show that electron-deficient conjugated π-systems bias the dispersion selectivity toward metallic SWNTs. Differentiation of semiconducting and metallic SWNT populations was carried out by a combination of UV/Vis-NIR absorption spectroscopy, Raman spectroscopy, fluorescence spectroscopy, and electrical conductivity measurements. These results provide new insight into the rational design of conjugated polymers for the selective dispersion of metallic SWNTs.
The synthesis and characterization of compounds derived from 9,9-dialkylfluorene and 1,4-dioxane[3,4-b]thiophene are described. The key step involves a modified Steglich reaction between 9,9-dialkyl-2,7-N,N′-diallyliminochloro-fluorene or 2,5-N,N′-diallyliminochloroethylenedioxythiophene and tert-BuOK (Route 1). Route 2 for bis(pyrrol-2-yl)dioxanethiophene, involving the reaction between 1,4-dioxane[2,3-c]thiophene and a dioxolanepropanol, is also a useful and general strategy. Molecular modeling studies indicate relationships between the molecular parameters (structure, ionization potential, atomic charges) of the synthesized moieties and their polymerization properties.
A series of bifunctional oligofluorene-cored carbazole dendrimers (GnFm, n = 1-3, m = 2-3) containing carbazole dendrons up to the third generation as end-caps were synthesized and characterized as non-doped solution-processed blue-light emitters and hole transporters for organic light-emitting diodes (OLEDs). Their optical, thermal, electrochemical, and electroluminescence properties were investigated. They exhibited a strong deep-blue fluorescence with solution fluorescence quantum yields (ΦF) of around 0.91-0.99 and formed morphologically stable amorphous thin films with glass transition temperatures as high as 273 °C. As blue emitters, solution-processed OLEDs with structure of ITO/PEDOT:PSS/GnFm/BCP/LiF:Al displayed a deep-blue emission (λELem = 415 nm, CIE = 0.17, 0.11) with a maximum luminance efficiency as high as 3.79 cd A-1 and a low turn-on voltage of 4.2 V. As hole transporters, solution-processed OLEDs with structure of ITO/PEDOT:PSS/GnFm/Alq3/LiF:Al showed a bright green emission (λELem = 520 nm, CIE = 0.30, 0.54) with a maximum luminance efficiency as high as 5.63 cd A-1 and a low turn-on voltage of 2.4 V. This journal is the Partner Organisations 2014.
A series of alternating conjugated copolymers based on thiadiazolo[3,4-g] quinoxaline units and different electron-donating units, such as fluorene, benzene and thiophene, with donor (D)-acceptor (A) alternating structures have been synthesized by palladium catalyzed Sonogashira condensation polymerization. The resulting copolymers P1, P2 and P3 were characterized by NMR, IR, gel permeation chromatography, thermogravimetric analysis and differential scanning calorimetry. Their optical and electronic properties can be facilely fine-modulated by adjusting the structures of different aromatic or heteroaromatic blocks. UV-visible absorption and cyclic voltammetry measurements indicate that all these copolymers have low band gaps due to the strong interaction between the donor and thiadiazolo[3,4- g]quinoxaline segments. Polymer P1 exhibits highest HOMO energy level, which can be expected to obtain high open-circuit voltage (Voc) from the fabricated PSCs. Polymer P3 based on thiophene and thiadiazolo[3,4-g]quinoxaline show smallest band gap and best absorption of sun light even in near-infrared (NIR) region. Preliminary studies imply that these copolymers can be used as efficient polymer donor materials in polymer solar cell applications.
Bis(fluorenyl)benzothiadiazole-cored carbazole dendrimers show high thermal and electrochemical stability, and great potential as solution processed hole-transporting non-doped green emitters for OLEDs. A pure green device with CIE coordinates of (0.27, 0.62) and high luminance efficiencies (up to 10.01 cd A-1) is achieved, respectively.
The invention discloses a self-repairing platinum metal gel and a preparation method and application thereof, and belongs to the field of self-repair metal gel materials. A self-healing platinum metal gel Pt-4 CHO, having the structure of Formula I, by performing a series of substitution reactions to the feedstock 2, 7 - dibromo - 9H - fluorene. With sonogashira-and CHO aldehyde group conversion into imine-based synthetic model molecule Pt 4 Pt 4 Pt-4 imine through Schiff base reaction, the prepared platinum metal gel has good self-repairing property, optical transparency, mechanical property and light-limiting property, and laser protection performance is further improved due to nonlinear scattering of laser in the gel.
The invention discloses a preparation method of 2,7-dibromo-9,9-dialkyl-1,6-binitro-fluorene and belongs to the field of organic chemical synthesis. The preparation method comprises the following steps: utilizing fluorene as a raw material and reacting with liquid bromine to obtain 2,7-dibromo-fluorene; then, dissolving the 2,7-dibromo-fluorene into a methylbenzene and NaOH water solution and reacting with hylogenated hydrocarbon to obtain 2,7-dibromo-9,9-dialkyl-fluorene; then, reacting the 2,7-dibromo-9,9-dialkyl-fluorene with ceric ammonium nitrate to obtain a final product of 2,7-dibromo-9,9-dialkyl-1,6-binitro-fluorene. By means of utilizing the ceric ammonium nitrate as a nitration reagent, the preparation method disclosed by the invention has the advantages of quick reaction speed,good selectivity, moderate reaction condition and small byproduct. Furthermore, two nitro groups are introduced into 1,6 sites of the flurene for the first time, so that obtained asymmetric binitro-fluorene can be applied to design synthesis of organic photoelectric materials.
Three novel AuI polyynes have been prepared in high yield by copolymerization between an AuI complex precursor and different ethynyl aromatic ligands. The investigation of their photophysical behavior has indicated that forming polyynes through polymerization not only maintains the high transparency of the corresponding AuI polyynes similar to those of their corresponding small molecular AuI acetylides, but also effectively enhances their triplet (T1) emission ability. Critically, owing to their enhanced T1 emission ability, all the AuI polyynes exhibit a stronger optical power limiting (OPL) ability against a 532 nm laser than the corresponding small molecular AuI acetylides. The AuI polyynes based on fluorene and triphenylamine ligands show even better OPL performance than the state-of-the-art OPL material C60, indicating their great potential in the field of laser protection. More importantly, in a prototype OPL device made by doping the fluorene-based AuI polyyne into a polystyrene (PS) solid matrix, substantially improved OPL activity has been observed compared with that in the solution, demonstrating its great potential for practical application. All these results have provided a new strategy to achieve a balance between high OPL activity and good transparency for OPL materials, representing a valuable attempt towards developing new OPL materials with high performance to cope with the key problems in the field of nonlinear optics.
Two series of new heterobimetallic Au(i)-Pt(ii) polyynes have been easily synthesized by cross-coupling under mild conditions. The absorption profiles of these two series of Au(i)-Pt(ii) polyynes are quite similar. However, the Au(i)-Pt(ii) polyynes with a 1,4-bis(diphenylphosphino)benzene ligand show stronger triplet (T1) emission and superior optical power limiting (OPL) performance than the corresponding Au(i)-Pt(ii) polyynes with a 1,3-bis(diphenylphosphino)propane ligand. Hence, the 1,4-bis(diphenylphosphino)benzene ligand is more effective than the 1,3-bis(diphenylphosphino)propane ligand for optimizing the transparency and OPL ability of OPL materials. When compared with the corresponding homometallic Pt(ii) polyynes, these heterobimetallic Au(i)-Pt(ii) polyynes display a blue shift in their absorption spectra, showing better transparency in the visible-light region. Besides, these heterobimetallic Au(i)-Pt(ii) polyynes show stronger OPL ability than their corresponding homometallic Pt(ii) polyynes as well as the state-of-the-art OPL material C60, demonstrating their enormous application potential in the nonlinear optics field. In brief, the introduction of Au(i) precursors with tetrahedral diphosphine ligands into the backbone of Pt(ii) polyynes can simultaneously achieve enhanced transparency and high OPL ability for OPL materials, providing a new strategy to optimize OPL materials.
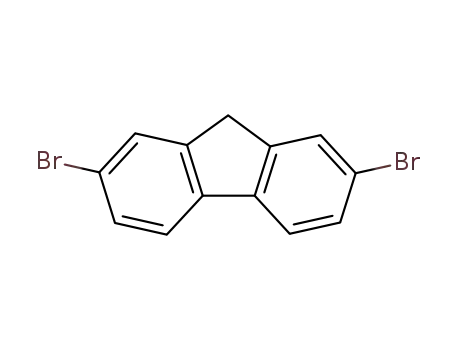
2,7-dibromo-9H-fluorene


1-dodecylbromide

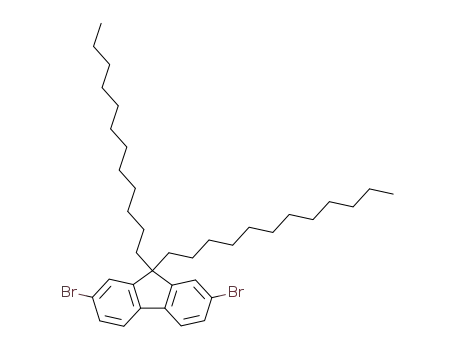
2,7-dibromo-9,9-di-n-dodecylfluorene
| Conditions | Yield |
|---|---|
|
With
potassium tert-butylate;
In
tetrahydrofuran;
at 0 - 20 ℃;
for 12h;
|
92% |
|
With
tetrabutylammomium bromide; water; sodium hydroxide;
In
dimethyl sulfoxide;
at 20 ℃;
for 3h;
|
87% |
|
With
tetrabutylammomium bromide; sodium hydroxide;
In
water; toluene;
at 80 ℃;
for 24h;
Inert atmosphere;
|
86.6% |
|
With
potassium tert-butylate;
In
N,N-dimethyl-formamide;
at 40 ℃;
for 18h;
|
84% |
|
With
tetrabutylammomium bromide; sodium hydroxide;
In
water; dimethyl sulfoxide;
at 20 ℃;
for 3h;
|
82% |
|
With
sodium hydroxide;
In
dimethyl sulfoxide;
at 90 ℃;
for 24h;
|
80.6% |
|
With
tetrabutylammomium bromide; sodium hydroxide;
In
water; dimethyl sulfoxide;
at 20 ℃;
for 3h;
|
78% |
|
2,7-dibromo-9H-fluorene; 1-dodecylbromide;
With
potassium hydroxide;
In
toluene;
for 1h;
Inert atmosphere;
With
tetrabutylammomium bromide;
In
toluene;
at 60 ℃;
for 12h;
Inert atmosphere;
|
63% |
|
With
tetrabutylammomium bromide; sodium hydroxide;
In
water; toluene;
Inert atmosphere;
|
32% |
|
With
N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide;
at 60 ℃;
for 12h;
|
|
|
With
potassium hydroxide;
In
water; toluene;
|
|
|
With
tetrabutylammomium bromide; potassium hydroxide;
In
water; dimethyl sulfoxide;
|
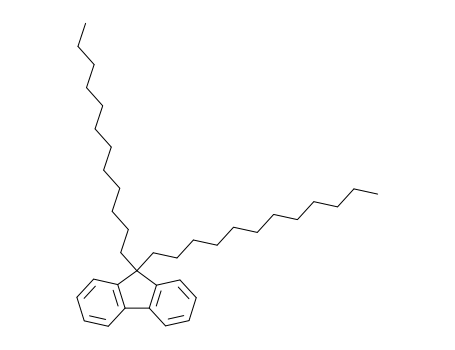
9,9-didodecyl-9H-fluorene


2,7-dibromo-9,9-di-n-dodecylfluorene
| Conditions | Yield |
|---|---|
|
With
bromine;
iodine;
In
dichloromethane;
at 20 ℃;
for 20h;
|
80% |

9,9-didodecyl-9H-fluorene
9H-fluorene

1-dodecylbromide

2,7-dibromo-9H-fluorene
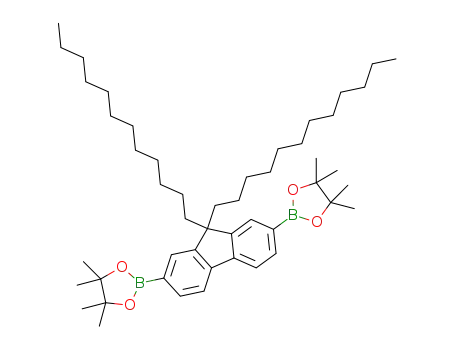
2,2'-(9,9-didodecyl-9H-fluorene-2,7-diyl)bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane)
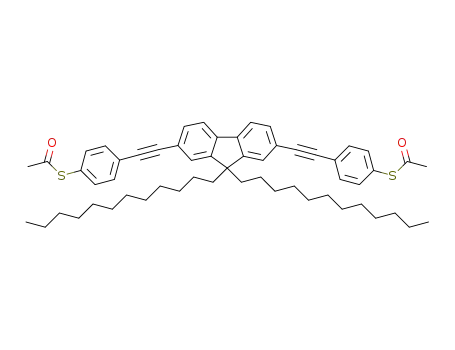
9,9-didodecyl-2,7-bis((4-(acetylthio)phenyl)ethynyl)fluorene
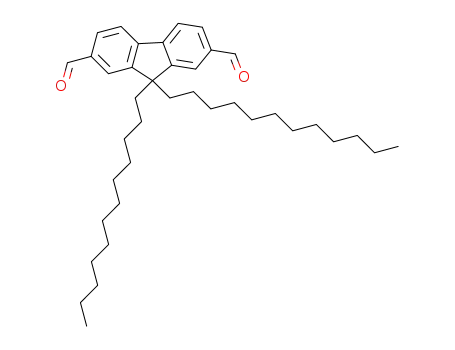
9,9-didodecylfluorene-2,7-dicarbaldehyde
CAS:1210469-11-6
Molecular Formula:C22H14BrN
Molecular Weight:372.3
CAS:5315-79-7
CAS:50-00-0