Your Location:Home >Products >Organic phosphines >Phenyl phosphines >37943-90-1
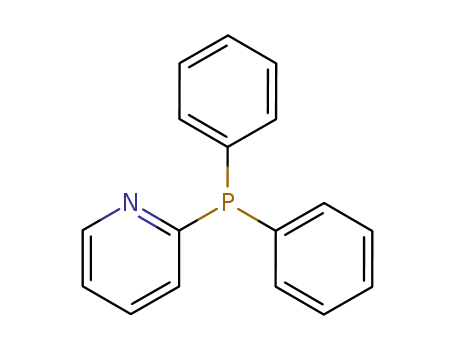

Product Details
Reaction
Ligand for the palladium-catalyzed distannylation of ortho-quinodimethanes Ligand for the palladium-catalyzed disilylation of o-quinodimethanes to synthesize 9- and 10-membered disilacarbocycles Ligand for the palladium-catalyzed alkoxycarbonylation of allenes
Chemical Properties
Yellow to orange crystalline powder
Uses
Diphenyl-2-pyridylphosphine is an organophosphorous compound that is a widely used mono-pyridylphosphine ligand in transition metal complexes for catalysis.
Uses
suzuki reaction
Uses
Ligand for metal-catalyzed carbonylations, hydration, dehydrogenative coupling, carbostannylation, distannylation, and silylation; reagent for Mitsunobu reaction.
InChI:InChI=1/C17H14NP/c1-3-9-15(10-4-1)19(16-11-5-2-6-12-16)17-13-7-8-14-18-17/h1-14H
The reaction of 2-pyridiyldiphenylphosphine (2) with tetrachloromethane and subsequent dehalogenation of the intermediate chloro phosphonium salt [(CDPPy2)Cl]Cl (3) with tris(1-pyrrolidyl)phosphine results in the formation of a new type of carbodiphosphorane N,C,N pincer ligand, sym-bis(2-pyridyl)tetraphenylcarbodiphosphorane, CDPPy2 (1). It crystallizes in a triclinic crystal system with a crystallographic point group of P1. This neutral double-ylidic N,C,N ligand is capable of stabilizing a wide range of metal coordination polyhedra, varying from square planar [(CDPPy2)PdCl]Cl (4), octahedral mer-[(CDPPy2)TiCl3] (5) and fac-[(CDPPy2)Cr(CO)3] (6) to trigonal-bipyramidal [(CDPPy2)MnCl2] (9) and [(CDPPy2)CoCl2] (10) complexes. Unprecedented dinuclear complexes are formed with molybdenum and nickel carbonyls. 1 reacts with [Mo(CO)3(NCMe)3] to form the symmetric κ3-N,C,N-[(CDPPy2)Mo(CO)3(μ-CO)Mo(CO)3] (7) with one bridging carbonyl next to a bridging central carbon atom with its two lone pairs. In contrast, an unsymmetrical coordination mode with only one coordinated pyridine is observed in κ2-N,C-[(CDPPy2)Ni(CO)(μ-CO)Ni(CO)2] (8). Carbodiphosphorane-based ligands are unique due to their σ,πfour-electron-donor character of the central carbon atom toward one metal and alternatively their 2σ four-electron-donor character toward two vicinal metal atoms.
We describe a general and efficient protocol for the synthesis of organophosphine compounds from phenols and phosphines (R2PH) via a metal-free C-O bond cleavage and C-P bond formation process. This approach exhibits broad substrate scope and excellent functional group tolerance. The synthetic utilities of this protocol were demonstrated by the synthesis of chiral ligands via the various transformations of cyano groups and their applications in asymmetric catalysis.
A simple synthetic method of triarylphosphine compounds by KOH-promoted P-Arylation reaction of aryl halides with diphenylphosphine is presented. Notably, this transformation could smoothly proceed with high yields under transition-metal-free and mild reaction conditions. In addition, this protocol is valuable for industrial application due to the convenient operation and readily accessible aromatic halides. A possible explanation of the reaction mechanism was proposed based on the experimental data.
Reduction of phosphine oxides into the corresponding phosphines using PhSiH3 as a reducing agent and Ph3C+[B(C6F5)4]? as an initiator is described. The process is highly efficient, reducing a broad range of secondary and tertiary alkyl and arylphosphines, bearing various functional groups in generally good yields. The reaction is believed to proceed through the generation of a silyl cation, which reaction with the phosphine oxide provides a phosphonium salt, further reduced by the silane to afford the desired phosphine along with siloxanes. (Figure presented.).
The invention aims to provide an aryl phosphine oxide compound as a raw material, wherein P=O keys are activated by an acid anhydride and alkali is continued. The preparation of the phosphine (III) compound is carried out under the action of a crown ether and a reducing agent. The method has the advantages of cheap and easily available raw materials, simple operation, high atomic economy and the like. Compared with a traditional reduction mode, the method is ingenious in design, waste emission is reduced, separation of intermediate products is omitted, and related reagents such as silicon hydrogen, aluminum, boron and the like with higher price can be avoided. And the reaction suitability is extensive.
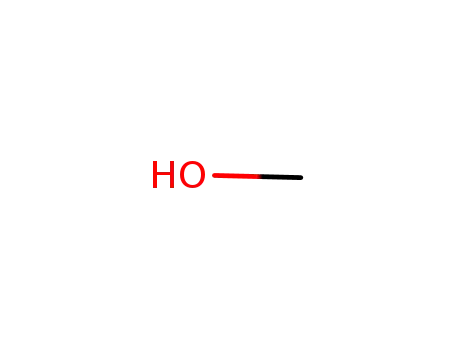
methanol

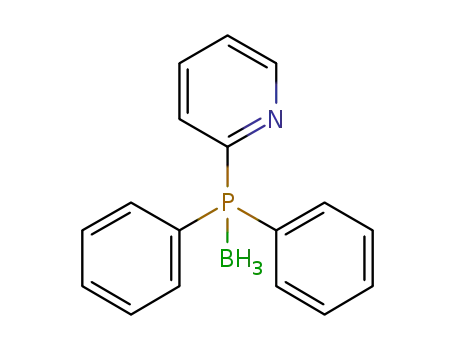
diphenyl(2-pyridyl)phosphane(P-B)borane(1:1)

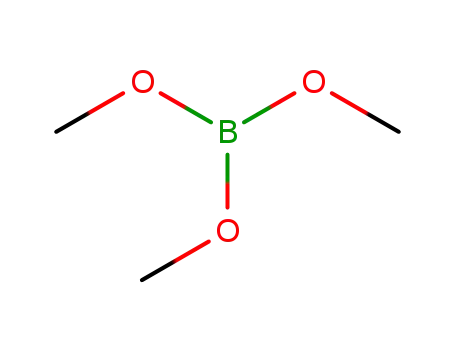
Trimethyl borate

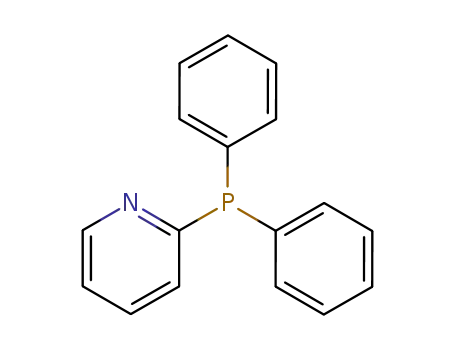
2-(diphenylphosphino)pyridine
| Conditions | Yield |
|---|---|
|
at 120 ℃;
Inert atmosphere;
Microwave irradiation;
|
100% |
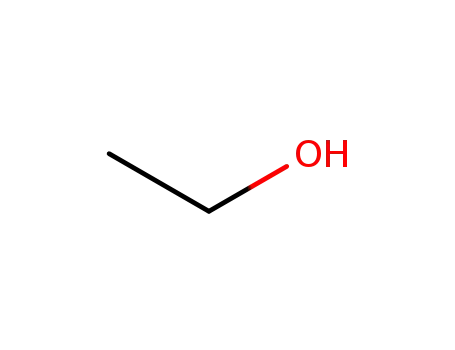
ethanol


diphenyl(2-pyridyl)phosphane(P-B)borane(1:1)

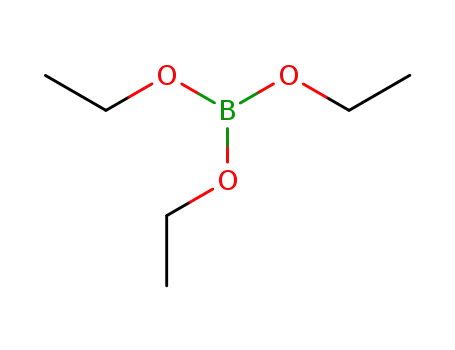
triethyl borate


2-(diphenylphosphino)pyridine
| Conditions | Yield |
|---|---|
|
Inert atmosphere;
Reflux;
|
97% |
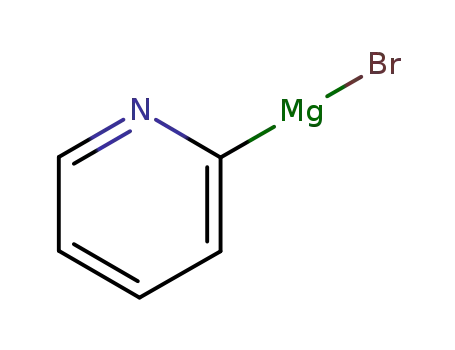
(pyridin-2-yl)magnesium bromide
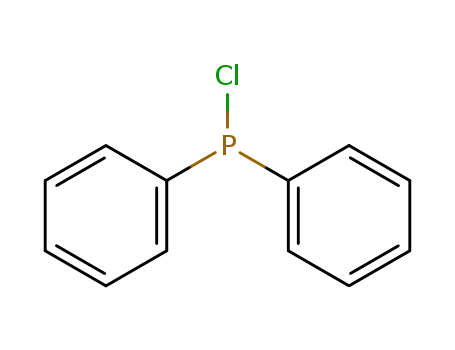
chloro-diphenylphosphine
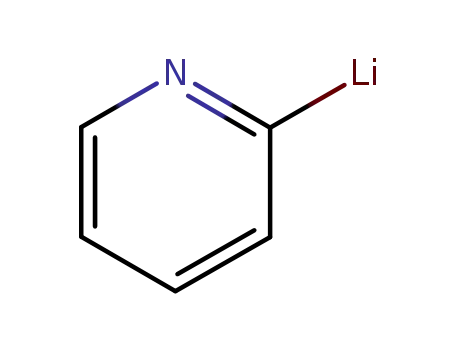
2-pyridyllithium
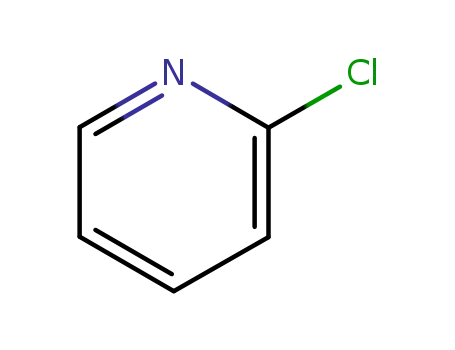
2-chloropyridine
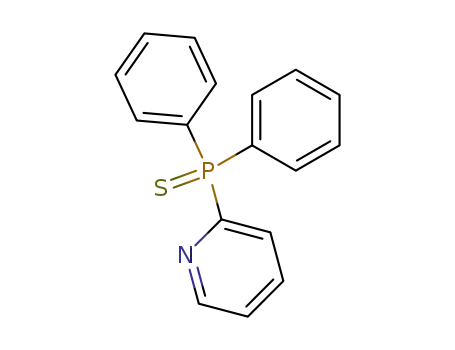
diphenyl-[2]pyridyl-phosphine sulfide
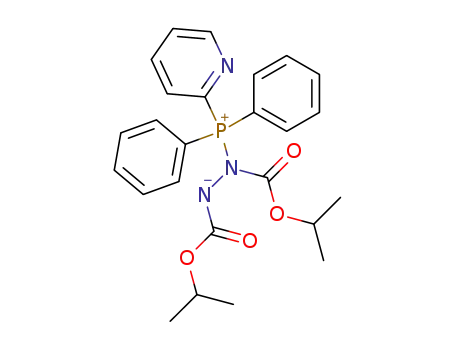
C25H28N3O4P
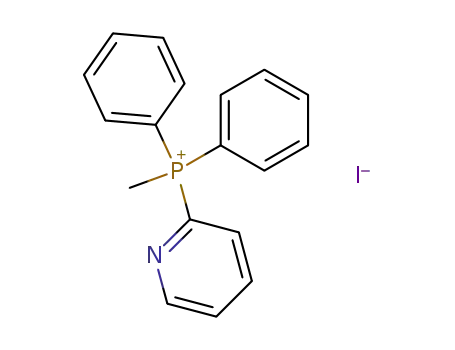
Methyl-diphenyl-pyridin-2-yl-phosphonium; iodide
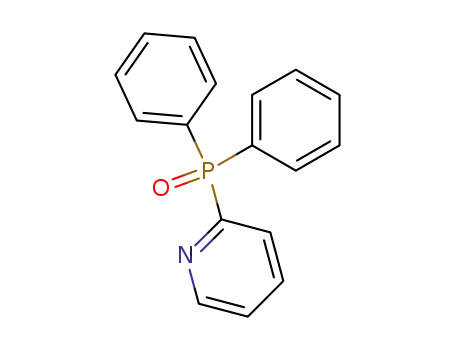
2-(diphenylphosphoryl)pyridine
CAS:300-57-2
CAS:240417-00-9
CAS:19999-87-2
CAS:739-58-2