Your Location:Home >Products >Organic phosphines >CyclohexyI phosphines >19999-87-2
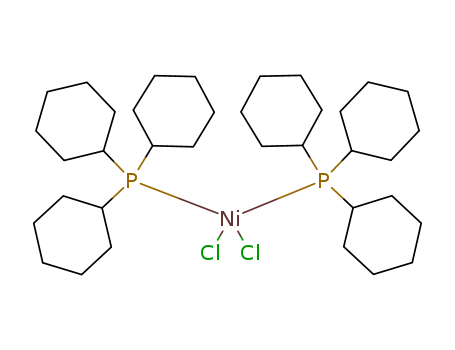

Product Details
Reaction
An approach to five-membered lactams from aliphatic amides and terminal acetylenes by nickel catalysis. Synthesis of biaryls through nickel-catalyzed Suzuki-Miyaura coupling of amides by carbon-nitrogen bond cleavage. Ni-catalyzed borylation of aryl fluorides via C-F cleavage. Nickeland palladium–catalyzed coupling of aryl fluorosulfonates with aryl boronic acids enabled by sulfuryl fluoride. Nickel-catalyzed one-pot synthesis of biaryls from phenols and arylboronic acids via C-O activation using TCT reagents.
Uses
Catalyst for:Dehydrobrominative polycondensationArylation reactionsCross-couplingSuzuki-miyaura cross-coupling reactionsKumada coupling of diaryl sulfates with Grignard reagentsOlefin dimerization
InChI:InChI=1/2C18H33P.2ClH.Ni/c2*1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;;;/h2*16-18H,1-15H2;2*1H;/q;;;;+2/p-2
The invention belongs to the field of catalyst preparation and application, and particularly relates to a catalyst and application thereof in synthesis of aromatic fluorine compounds. The nickel catalyst is dichloro-bis-(tri-cyclohexylphosphine) nickel, and the molecular formula of the nickel catalyst is Ni (Py3) 2Cl2. The nickel catalyst is applied to catalyzing inorganic fluoride to replace aromatic chloride to synthesize fluoride. The catalyst has the advantages of easily available reagents, simple catalyst synthesis, simple operation conditions, low reaction temperature, high reaction yield and less time consumption.
Described herein are nickel pre-catalysts and related compositions and methods. The nickel pre-catalysts may be activated to form catalysts which may be utilized in organic reactions.
A series of air-stable nickel complexes of the form L2Ni(aryl) X (L = monodentate phosphine, X = Cl, Br) and LNi(aryl)X (L = bis-phosphine) have been synthesized and are presented as a library of precatalysts suitable for a wide variety of nickel-catalyzed transformations. These complexes are easily synthesized from low-cost NiCl2·6H2O or NiBr 2·3H2O and the desired ligand followed by addition of 1 equiv of Grignard reagent. A selection of these complexes were characterized by single-crystal X-ray diffraction, and an analysis of their structural features is provided. A case study of their use as precatalysts for the nickel-catalyzed carbonyl-ene reaction is presented, showing superior reactivity in comparison to reactions using Ni(cod)2. Furthermore, as the precatalysts are all stable to air, no glovebox or inert-atmosphere techniques are required to make use of these complexes for nickel-catalyzed reactions.
The objective of this study was the design of a scaleable process for the synthesis of 3-4 mol of α-terthienyl from 2,5-dibromothiophene and thienylmagnesium bromide in a 10-L stirred tank reactor. In THF the Grignard reagent, thienylmagnesium bromide, was readily formed from 2-bromothiophene and magnesium. To avoid crystallization the maximal concentration was limited to 1.4 M. Furthermore, the novel combination of THF and NiCl2[bis(diphenylphosphino)benzene] allows for fast double coupling of the Grignard reagent with 2,5-dibromothiophene. The concentration of catalyst could be limited to 0.5 mol % based on the amount of 2,5-dibromothiophene. An adapted workup procedure was developed, in which n-octane was used to separate the magnesium salts from the desired product. The reaction was performed in a (semi)batch-wise operated reactor. A global model for the coupling step proved to predict the results at 0.1-, 1-, and 10-L scales very accurately. The heat of reaction evolved in the coupling step was valorized and could be handled easily. Mixing of the feed stream and the reactor content proved to be another important factor in the scaling-up of the α-terthienyl synthesis.
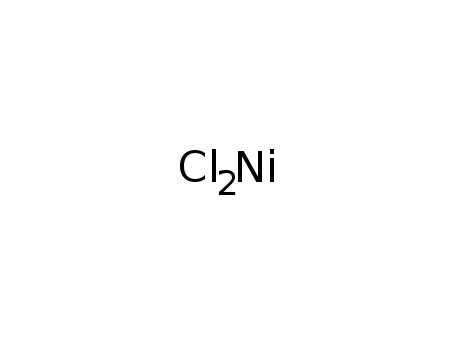
nickel dichloride

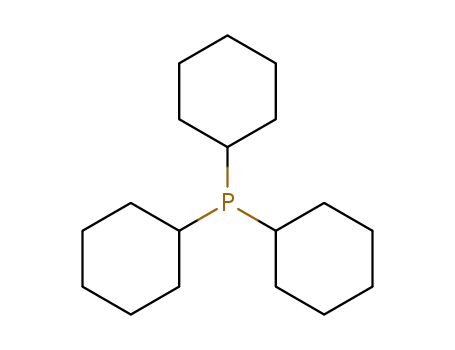
tricyclohexylphosphine

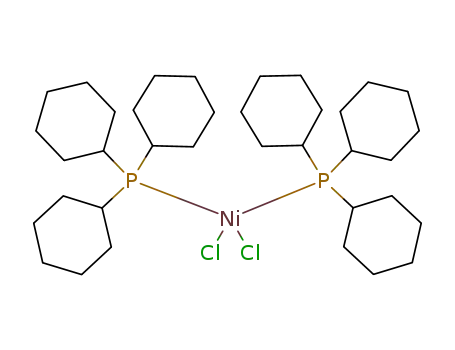
bis(tricyclohexylphosphine)nickel(II) dichloride
| Conditions | Yield |
|---|---|
|
In
ethanol;
at 80 ℃;
for 1.5h;
Inert atmosphere;
|
83.6% |
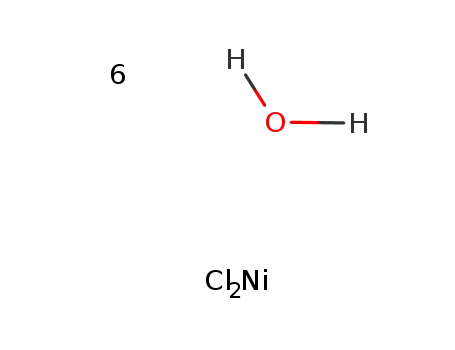
nickel(II) chloride hexahydrate


tricyclohexylphosphine


bis(tricyclohexylphosphine)nickel(II) dichloride
| Conditions | Yield |
|---|---|
|
In
ethanol;
at 80 ℃;
for 1h;
Sealed tube;
Inert atmosphere;
|
97% |
|
In
ethanol;
mixt. of Ni-complex in EtOH with phosphine, refluxing overnight;
|
|
|
In
methanol;
for 24h;
Inert atmosphere;
Reflux;
|

tricyclohexylphosphine

nickel dichloride
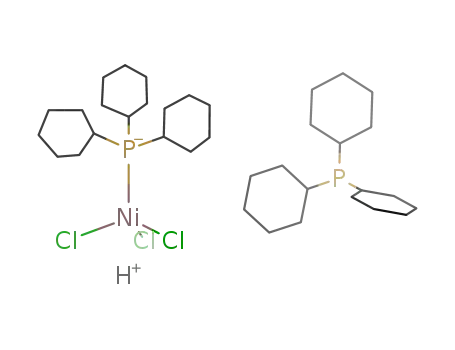
{Cy3PH}{(Cy3P)NiCl3}
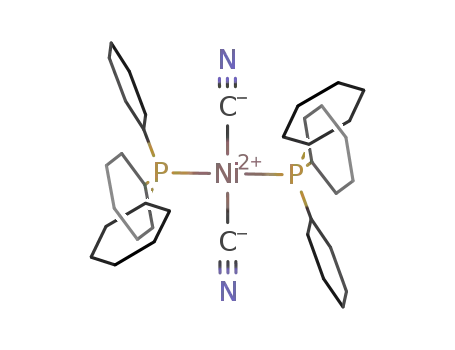
trans-dicyanobis(tricyclohexylphosphine)nickel(II)
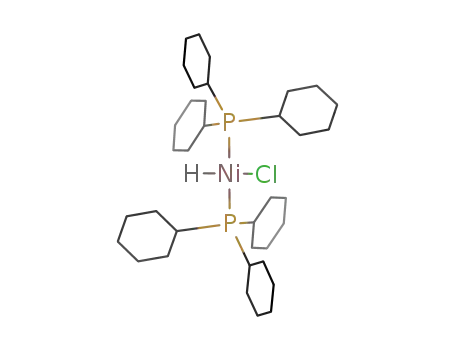
Ni(PCy3)2HCl
trans-bis(tricyclohexylphosphine)(2-methylphenyl)nickel(II) chloride
CAS:439791-57-8
CAS:108756-88-3
CAS:37943-90-1