Your Location:Home >Products >OLED intermediates >Fluorenes >1548450-59-4


Product Details
The present invention describes amines with dibenzofuran or dibenzothiophene groups in combination with carbazole, especially for use as triplet matrix materials in organic electroluminescent devices. The invention further relates to a process for preparing the compounds of the invention and to electronic devices comprising these compounds.
The invention discloses a production method of 4-bromo-9-methyl-9'-phenylfluorene, and belongs to the field of organic chemical synthesis. The production method includes taking 2,7-di-tert-butylfluorene as a starting material, synthesizing 2,7-di-tert-but
The present invention concerns particular fluorenes, the use of the compound in an electronic device, and an electronic device containing at least one of these compounds. The present invention further concerns a method for producing the compound and a for
A compound of formula (I) or (III) (Formulae (I), (III)) wherein: one Y is a substituent R1 bound directly to the fluorene unit of formula (I) by an sp3-hybridised carbon atom; the other Y is an aryl or heteroaryl group Ar1 that may be unsubstituted or substituted with one or more substituents; Ar2 is an arylene or heteroarylene group; R2 is a substituent; b is 0, 1, 2, 3 or 4; c is 0, 1, 2 or 3; and X is a group of formula (II): (Formula (II)) wherein Z is O or S; R3 independently in each occurrence is a substituent; each x is independently 0, 1, 2 or 3; and * is a bond to the fluorene unit of formula (I).The compounds may be used as host materials for phosphorescent dopants in organic light-emitting devices.
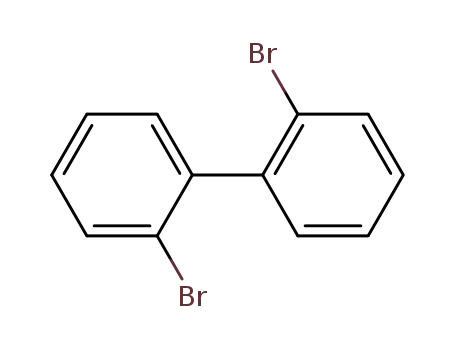
2,2'-dibromobiphenyl

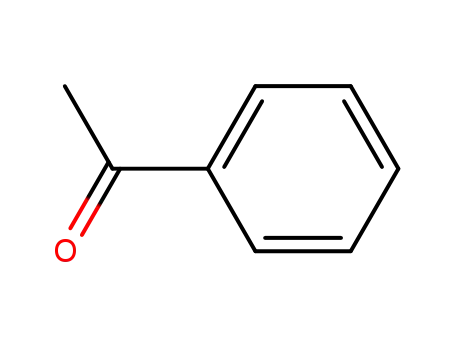
acetophenone

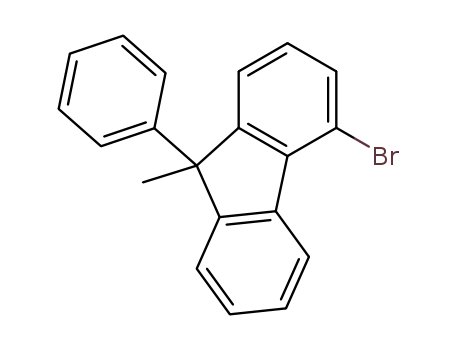
4‐bromo‐9‐methyl‐9‐phenylfluorene
| Conditions | Yield |
|---|---|
|
2,2'-dibromobiphenyl;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 - -70 ℃;
for 2h;
Heating;
acetophenone;
In
tetrahydrofuran; hexane;
at -70 - 20 ℃;
|
80% |
|
2,2'-dibromobiphenyl;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 - -70 ℃;
for 1h;
acetophenone;
In
tetrahydrofuran; hexane;
at -70 - 20 ℃;
With
hydrogenchloride; acetic acid;
In
water;
at 75 ℃;
for 6h;
|
80% |
|
2,2'-dibromobiphenyl;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 - -70 ℃;
for 2h;
Inert atmosphere;
acetophenone;
In
tetrahydrofuran; hexane;
at -70 ℃;
|
80% |
|
2,2'-dibromobiphenyl;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 - -70 ℃;
for 1h;
acetophenone;
In
tetrahydrofuran; hexane;
at -70 - 20 ℃;
|
77% |
|
With
n-butyllithium; acetic acid;
In
tetrahydrofuran; hexane;
|
|
|
2,2'-dibromobiphenyl;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 - -70 ℃;
for 2h;
acetophenone;
In
tetrahydrofuran; hexane;
at -70 - 20 ℃;
|
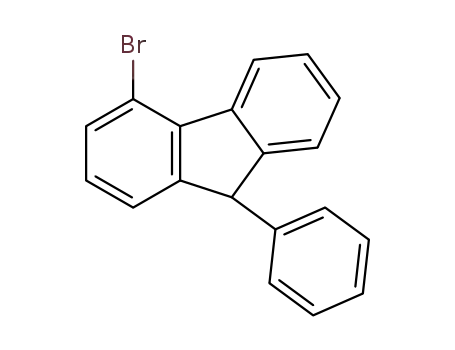
4-bromo-9-phenylfluorene

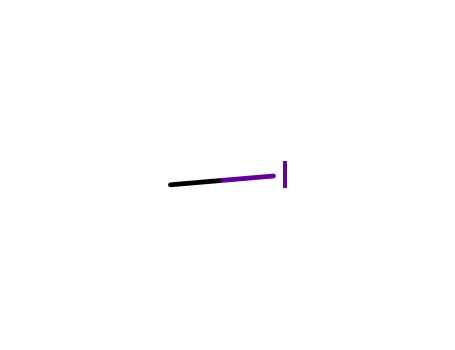
methyl iodide

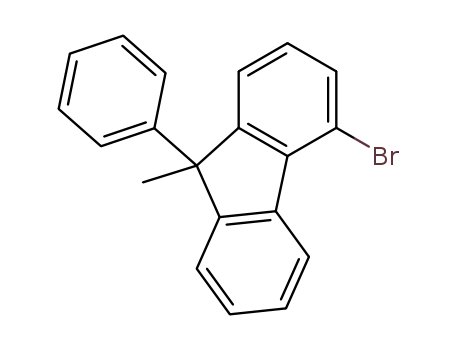
4‐bromo‐9‐methyl‐9‐phenylfluorene
| Conditions | Yield |
|---|---|
|
With
potassium tert-butylate;
In
tetrahydrofuran;
at -20 - 20 ℃;
for 16h;
Inert atmosphere;
|
42 g |
|
In
dimethyl sulfoxide;
at 5 ℃;
Temperature;
|
142.7 g |

2,2'-dibromobiphenyl

acetophenone

methyl iodide
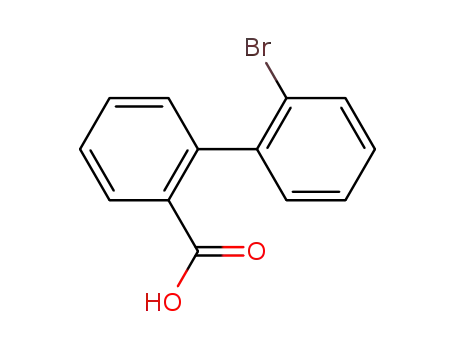
2'-bromo-[1,1'-biphenyl]-2-carboxylic acid
CAS:333432-28-3
Molecular Formula:C15H15BO2
Molecular Weight:238.09
CAS:1081-34-1
CAS:736928-22-6
CAS:1609484-45-8