Your Location:Home >Products >OLED intermediates >Fluorenes >736928-22-6
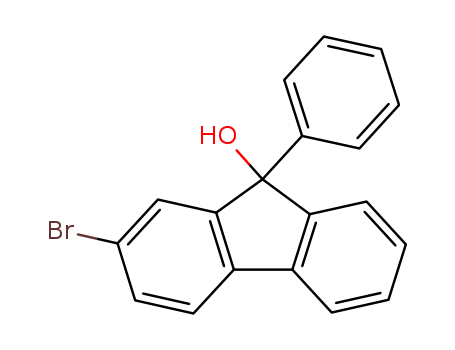

Product Details
InChI:InChI=1/C19H13BrO/c20-14-10-11-16-15-8-4-5-9-17(15)19(21,18(16)12-14)13-6-2-1-3-7-13/h1-12,21H
A new bipolar host material based on triphenylamine, fluorene and 1,2-diphenyl-1H-benzo[d]imidazole moieties, N,N-diphenyl-4-(9-phenyl-2-(4-(1-phenyl-1H-benzo[d]imidazol-2-yl)phenyl)-9H-fluoren-9-yl)aniline (DPPBIPFA), was designed and synthesized. The as-synthesized material was well characterized by1H and13C NMR spectroscopy, high-resolution mass spectrometry and thermogravimetric analysis, respectively. The photophysical and electrochemical properties of the material were also studied. The material exhibited an excellent thermal stability (Td= 475 °C), electrochemical stability and high triplet energy (2.68 eV). A green phosphorescent organic light-emitting diode (PhOLED) device based on DPPBIPFA as the host material and Ir(ppy)3as the dopant was fabricated, which displayed favorable electrophosphorescent properties with a turn-on voltage of 3.75 V, a maximum brightness of 1685 cd/m2and a maximum current efficiency of 4.26 cd/A.
A cyclic tension organic material, characterized in that a cyclic molecule having a ring tension and a structural rigidity is constructed based on fluorene or fluorene, wherein n is a natural number of 1-10 and R is a natural number in the general formula
The present specification relates to an ink composition including: a compound represented by Formula 1; and a solvent represented by the Formula 2, and a method for manufacturing an organic light emitting device formed by using the ink composition.
The invention discloses a preparation method of 2 - amino -9 and 9 - diphenyl fluorene, which comprises the following steps: taking 9 - fluorenone as a raw material, and carrying out bromination reaction to obtain 2 - bromo -9 - fluorenone. 2 - Bromo -9 - fluorenone and bromobenzene formative reagent are reacted to obtain 2 - bromo -9 - phenyl -9 - hydroxyl - fluorene. 2 - Bromo -9 - phenyl -9 - hydroxy - fluorene was subjected to an alkylation reaction with benzene to give 2 - bromo -9, 9 - diphenylfluorene. 2 - Bromo -9, 9 - diphenylfluorene and cuprous oxide, palladium acetate (II) and N - methylpyrrolidinone were reacted in liquid ammonia to give 2 - amino -9, 9 - diphenylfluorene. The preparation method is used for preparing 2 - amino -9 and 9 - diphenylfluorene, the used preparation is easy to obtain, the technological process is simple, the product yield is high, the production cost is reduced, 2 - amino -9 and 9 - diphenyl fluorene are produced.
The present specification relates to a compound, a coating composition including the same, and an organic light emitting device.
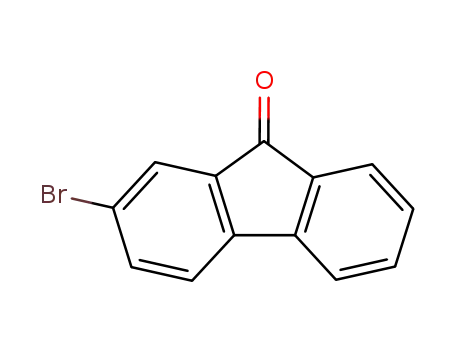
2-bromofluoren-9-one

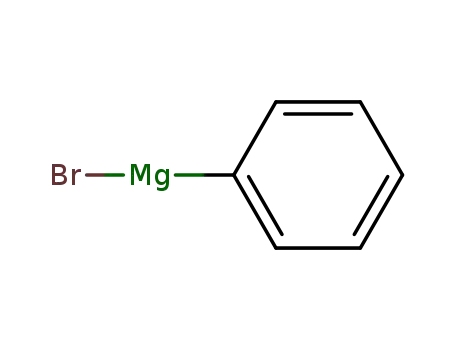
phenylmagnesium bromide

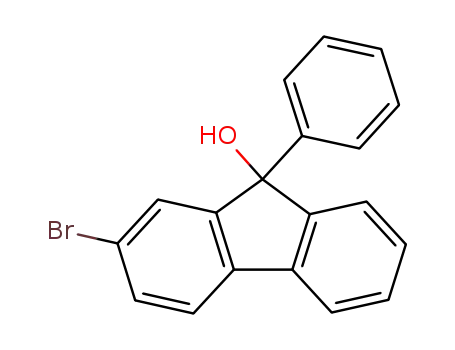
2-bromo-9-phenyl-9-hydroxyfluorene
| Conditions | Yield |
|---|---|
|
In
tetrahydrofuran;
at 0 ℃;
for 0.333333h;
|
100% |
|
In
diethyl ether;
at 0 ℃;
for 12h;
Inert atmosphere;
|
95% |
|
In
tetrahydrofuran;
at -78 - 40 ℃;
for 5.5h;
|
90% |
|
In
tetrahydrofuran;
at 0 - 20 ℃;
for 12h;
|
89% |
|
In
tetrahydrofuran;
at 0 - 20 ℃;
for 12h;
|
89% |
|
In
tetrahydrofuran;
at -78 - 20 ℃;
Inert atmosphere;
|
89% |
|
In
tetrahydrofuran;
at 20 ℃;
for 12h;
|
89% |
|
With
water;
In
tetrahydrofuran;
at 80 ℃;
for 5h;
Inert atmosphere;
|
79% |
|
2-bromofluoren-9-one; phenylmagnesium bromide;
In
tetrahydrofuran;
for 0.333333h;
With
triethylsilane; trifluoroacetic acid;
In
dichloromethane;
at 20 ℃;
|
61% |
|
In
tetrahydrofuran; diethyl ether;
at 0 ℃;
for 1h;
|
|
|
With
water; ammonium chloride;
In
tetrahydrofuran;
at 0 ℃;
|
|
|
In
tetrahydrofuran;
at 0 ℃;
|
|
|
In
tetrahydrofuran;
at 60 ℃;
for 24h;
|
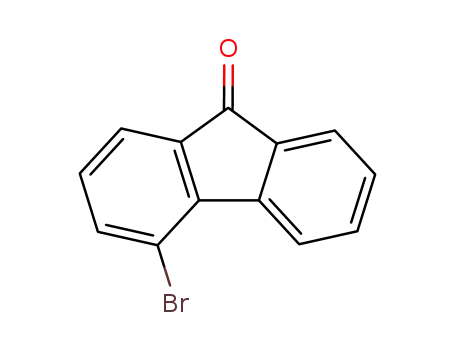
4-bromo-9H-fluorene-9-one

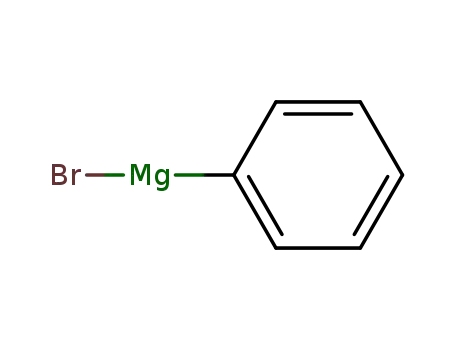
phenylmagnesium bromide


2-bromo-9-phenyl-9-hydroxyfluorene
| Conditions | Yield |
|---|---|
|
In
tetrahydrofuran;
at 0 ℃;
for 0.333333h;
|
100% |

2-bromofluoren-9-one
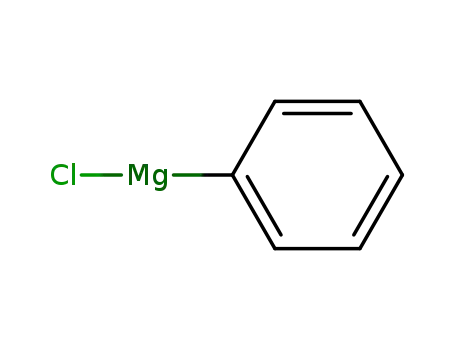
phenylmagnesium chloride
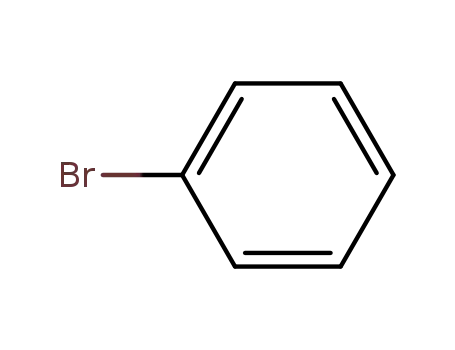
bromobenzene
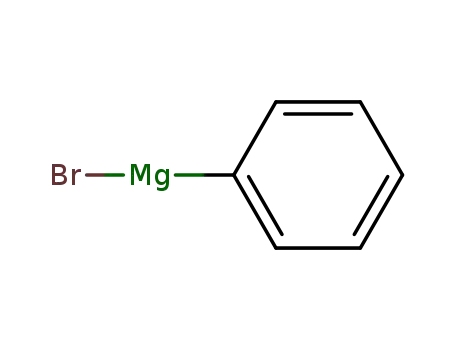
phenylmagnesium bromide

2-bromo-9,9-diphenyl-9H-fluorene
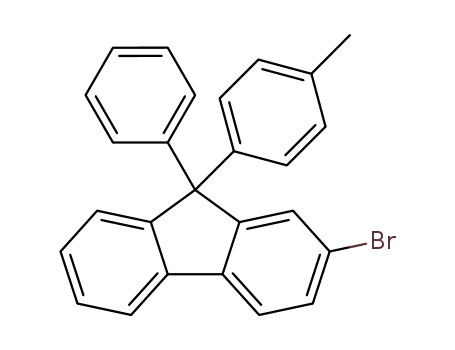
2-bromo-9-(4-methylphenyl)-9-phenyl-9H-fluorene
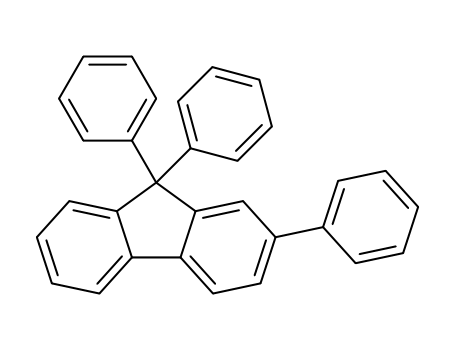
C31H22

C31H20Br2
CAS:854952-58-2
CAS:51792-34-8
CAS:99586-26-2
CAS:1548450-59-4