Your Location:Home >Products >OLED intermediates >Thiophenes >34722-01-5
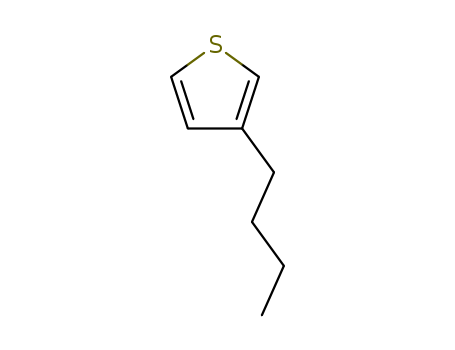

Product Details
Chemical Properties
Colorless liquid
General Description
3-Butylthiophene is an alkylthiophene.
InChI:InChI=1/C8H12S/c1-2-3-4-8-5-6-9-7-8/h5-7H,2-4H2,1H3
This article deals with the Kumada Catalyst Transfer Polycondensation (KCTP) of 4,7-dioctylbenzo[2,1-b:3,4-b']dithiophene (BDP-Oct) using Ni(II) catalyst or In/cat combination. A combination of MALDI MS, GPC, and 31P NMR spectroscopy is used to reveal the failure of the KCTP of this particular monomer. Intermolecular transfer reactions to monomer appeared to prevent the formation of polymer. This result is remarkable, since isomeric benzo[1,2-b:4,5-b']dithiophene polymerizes in a controlled way. The presence of a "non-aromatic double bond" in annulated monomers is discussed.
Nitroxide-containing organic radical polymers (ORPs) have captured attention for their high power and fast redox kinetics. Yet a major challenge is the polymer's aliphatic backbone, resulting in a low electronic conductivity. Recent attempts that replace the aliphatic backbone with a conjugated one have not met with success. The reason for this is not understood until now. We examine a family of polythiophenes bearing nitroxide radical groups, showing that while both species are electrochemically active, there exists an internal electron transfer mechanism that interferes with stabilization of the polymer's fully oxidized form. This finding directs the future design of conjugated radical polymers in energy storage and electronics, where careful attention to the redox potential of the backbone relative to the organic radical species is needed.
Herein, the optical properties of thiophene-functionalized quadrupolar carbo-benzenes and a benzenic parent, of generic structure Th–C[tbnd]C–[core]–C[tbnd]C–Th, Th = R2C4HS, are comparatively investigated. Beyond the previously unknown dioctylthienylethynylbenzene (core = p-C6H4, R = nOct), two bis-dialkylthienylethynyl-carbo-benzenes (core = C18Ph4, R = nOct, nBu) are envisaged for the unique “carbo-aromatic” character of the C18 macrocycle. The three targets were synthesized from the corresponding ethynylthiophenes in 47, 20 and 10% yield, respectively, then characterized by classical methods such as NMR spectroscopy, and X-ray crystallography for one of the carbo-benzenes. Regarding linear and nonlinear optical properties, our results show that the carbo-merization induces a significant shift to lower energies of the one-photon electronic excitations accompanied by an 8-fold increase of the molar extinction coefficient compared to the parent molecule. Intriguingly, these excitations lead to a broad band of photoluminescence comprising decay transitions of the type S1 → S0 but also of the type S2 → S0. This phenomenon of emission from higher excited states, which is contrary to Kasha's rule, is assigned to - or revealed by - a reduction of the internal conversion efficiency between S2 and S1. Two-photon induced transitions are also enhanced, the two-photon absorption cross-section (σ2PA) being in average five times larger for the carbo-benzenes than for their benzene parent in the wavelength range 650–950 nm, with a maximum of σ2PA = 1430 GM (1 GM = 10?50 cm4 s/photon). Beyond a moderate nonlinearity, this comparative study provides quantitative insights about the way carbo-merization or insertion of a π-conjugated macrocycle between chromophoric functions (here thiophene rings) can tune optical properties of organic molecules. The optical properties of the bis-dialkylthienylethynyl-carbo-benzenes are also discussed in regard of recent reports on organic chromophores based on other types of π-conjugated macrocyclic cores.
A series of organometallic complexes [Cl(dppe)2Ru?C≡C-(3-R?C4H2S)-C≡C?Ru(dppe)2Cl] (3-R-C4H2S=3-substituted thienyl moiety; R=?H, ?C2H5, ?C3H7, ?C4H9, ?C6H13, ?OMe, ?CN in 5 a–5 g respectively) have been synthesized by systematic variation of 3-substituents at the thienylethynyl bridging unit. The diruthenum(II) wire-like complexes (5 a–5 g) have been achieved by the reaction of thienylethynyl bridging units, HC≡C-(3-R-C4H2S)-C≡CH (4 a–4 g) with cis-[Ru(dppe)2Cl2]. The wire-like diruthenium(II) complexes undergo two consecutive electrochemical oxidation processes in the potential range of 0.0 - 0.8 V. Interestingly, the wave separation between the two redox waves is greatly influenced by the substituents at the 3-position of the thienylethynyl. Thus, the substitution on 3-position of the thienylethynyl bridging unit plays a pivotal role for tuning the electronic properties. To understand the electronic behavior, density functional theory (DFT) calculations of the selected diruthenium wire-like complexes (5 a–5 e) with different alkyl appendages are performed. The theoretical data demonstrate that incorporation of alkyl groups to the thienylethynyl entity leaves unsymmetrical spin densities, thus affecting the electronic properties. The voltammetric features of the other two Ru(II) alkynyl complexes 5 f and 5 g (with ?OMe and ?CN group respectively) show an apparent dependence on the electronic properties. The electronic properties in the redox conjugate, (5 a+) with Kc of 3.9×106 are further examined by UV-Vis-NIR and FTIR studies, showing optical responses in NIR region along with changes in “?Ru?C≡C?“ vibrational stretching frequency. The origin of the observed electronic transition has been assigned based on time-dependent DFT (TDDFT) calculations.
Direct palladium-catalysed cross-couplings between organolithium reagents and (hetero)aryl halides (Br, Cl) proceed fast, cleanly and selectively at room temperature in air, with water as the only reaction medium and in the presence of NaCl as a cheap additive. Under optimised reaction conditions, a water-accelerated catalysis is responsible for furnishing C(sp3)–C(sp2), C(sp2)–C(sp2), and C(sp)–C(sp2) cross-coupled products, in competition with protonolysis, within a reaction time of 20 s, in yields of up to 99 %, and in the absence of undesired dehalogenated/homocoupling side products even when challenging secondary organolithiums serve as the starting material. It is worth noting that the proposed protocol is scalable and the catalyst and water can easily and successfully be recycled up to 10 times, with an E-factor as low as 7.35.
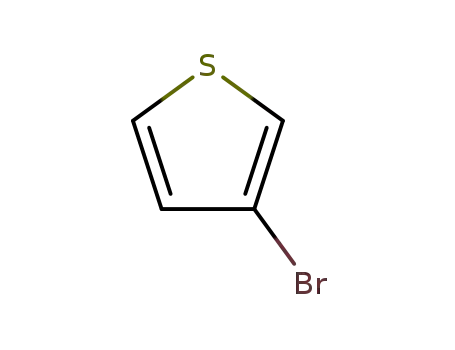
3-Bromothiophene

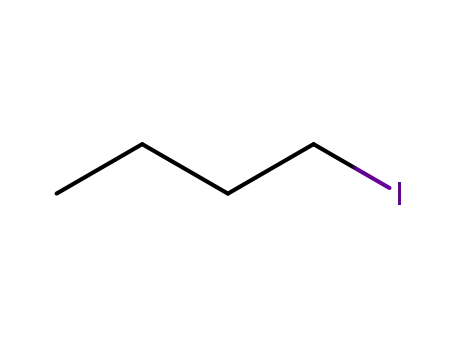
1-iodo-butane

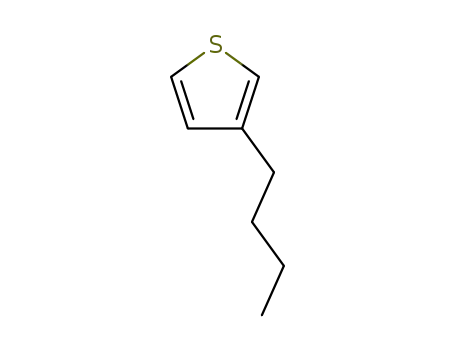
3-butylthiophene
| Conditions | Yield |
|---|---|
|
With
N,N,N,N,-tetramethylethylenediamine; di-tert-butyl[dichloro({di-tert-butyl[4-(dimethylamino)phenyl]phosphaniumyl})palladio][4-(dimethylamino)phenyl]phosphanium; zinc;
In
water;
at 20 ℃;
for 24h;
Inert atmosphere;
|
22% |

3-Bromothiophene

1-bromo-butane


3-butylthiophene
| Conditions | Yield |
|---|---|
|
1-bromo-butane;
With
magnesium;
In
tetrahydrofuran;
at 20 ℃;
for 1h;
Schlenk technique;
3-Bromothiophene;
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
In
tetrahydrofuran;
for 15h;
Schlenk technique;
Heating;
|
90% |
|
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II); magnesium;
1.) Et2O, 2.) heating, 15 h;
|
71.4% |
|
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II); magnesium;
Multistep reaction;
|
|
|
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II); magnesium;
Yield given. Multistep reaction;
1.) ether, 2.) ether, reflux, 15 h;
|
|
|
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II); magnesium;
Yield given. Multistep reaction;
1.) diethyl ether, room temperature, 1 h, 2.) diethyl ether, room temperature, overnight;
|
|
|
With
Cl2Ni(dppp); magnesium;
Yield given. Multistep reaction;
1.) ether, room temperature, 1 h, 2.) ether, room temperature, overnight;
|
|
|
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II); magnesium;
In
tetrahydrofuran;
for 24h;
Reflux;
|
Tetrahydrothiophen-3-one
n-butyl magnesium bromide

3-Bromothiophene

1-bromo-butane
2-bromo-3-(n-butyl)thiophene

3,3'-dibutyl-2,2'-bithiophene
diphenyl(pyridin-4-yl)methanol
4,4'-dibutyl-2,2'-bithiophene
CAS:4805-22-5
CAS:145543-82-4
CAS:825-55-8