Your Location:Home >Products >OLED intermediates >Thiophenes >825-55-8
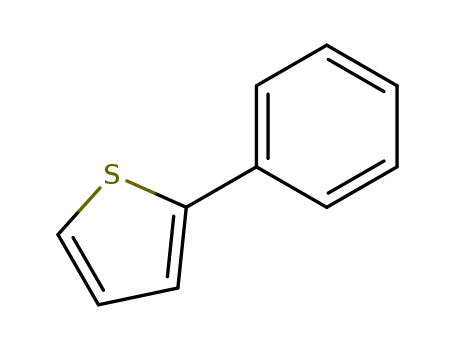

Product Details
Chemical Properties
white to light beige crystalline low melting mass
Synthesis Reference(s)
Tetrahedron, 38, p. 3347, 1982 DOI: 10.1016/0040-4020(82)80117-8Tetrahedron Letters, 25, p. 453, 1984 DOI: 10.1016/S0040-4039(00)99909-X
InChI:InChI=1/C10H8S/c1-2-5-9(6-3-1)10-7-4-8-11-10/h1-8H
-
The reaction of 1,2-bis(diphenylphosphino)ethane (dppe) with various ketones in acetone produces the new phosphonium salts [RC(O)CH 2PPh2(CH2)2PPh2CH 2C(O)R]X2 (R = 2-naphtyl, X
CoPd bimetallic nanoparticles (NPs) were successfully encapsulated in bamboo-like N-doped mesoporous carbon (bNMC) via a facile one-pot method through combination of dissolving chelation and carbonization reactions. The morphology, structure and composition of CoPd-bNMC was verified by detailed characterization including SEM, TEM, EDX, XPS, XRD, N2 adsorption–desorption, VSM, and ICP. The as-prepared CoPd/bNMC showed excellent activity and selectivity for Suzuki coupling under mild and green condition. Though the Pd content of CoPd/bNMC was half of Pd/bNMC, the catalytic performance of CoPd/bNMC was almost the same with Pd/bNMC, which was caused by its special bimetallic alloy structure, high BET surface area and pore volume. Importantly, CoPd/bNMC with magnetic property can be separated using external magnetic field and reused for five consecutive runs in the reaction of Suzuki crossing without significant loss of activity. It was found the Pd content only showed slight loss (2.3?wt.%) after five reused reactions, which was because CoPd bimetallic NPs were inside the bamboo-like N-doped mesoporous carbon.
In this study, magnetic nitrogen-doped carbon (MNC) was fabricated through facile carbonization and activation of natural silk cocoons containing nitrogen and then combined with Fe3O4 nanoparticles to create a good support material for palladium. Palladium immobilization on the support resulted in the formation of magnetic nitrogen-doped carbon-Pd (MNC-Pd). The prepared heterogeneous catalyst was well characterized using FT-IR, TGA, EDX, FE-SEM, XRD, VSM, and ICP-OES techniques. Thereafter, the synthesis of biaryl compounds was conducted to investigate the catalyst performance via the reaction of aryl halides and phenylboronic acid. Further, the catalyst could be used and recycled for six consecutive runs without any significant loss in its activity.
Carbon nanotube-supported palladium nanoparticles prepared by a supercritical fluid deposition method show high activities for catalyzing Suzuki coupling reactions, and the catalysts can be recycled and reused at least six times without losing activity. Copyright Taylor & Francis Group, LLC.
The new metalloligand ferrocenylbis(phosphonite), [Fe(C5H 4PR2)2 (R = OC6H3(OMe-o) (C3H5-p))], (2), is synthesized by the reaction of bis(dichlorophosphino)ferrocene Fe(C5H4PCl 2)2 (1) with 4-allyl-2-methoxyphenol. The reactions of 2 with H2O2 and elemental sulfur or selenium afforded bischalcogenides, [Fe{C5H4P(E)(OC6H 3(OMe-o)(C3H5-p))2}2] (3, E = O; 4, E = S; 5, E = Se), in good yield. The bis(phosphonite) reacts with group 6 metal carbonyls and group 10 metal dichloride precursors to produce the chelate complexes [{M(CO)4}Fe{C5H4P(OC 6H3(OMe-o)(C3H5-p)) 2}2}] (6, M = Mo; 7, M = W) and [(MCl2) Fe{C5H4P(OC6H3(OMe-o)(C 3H5-p))2}2] (8, M = Pd; 9, M = Pt). The palladium(ii) complex [(PdCl2)Fe{C5H 4P(OC6H3(OMe-o)(C3H 5-p))2}2] (8) is an efficient catalyst for the Suzuki-Miyaura cross-coupling reactions (TON up to 1.5 × 105). The reaction of 2 with one equivalent of [RuCl2(η6-p- cymene)]2 yielded the binuclear complex [{Ru2Cl 4(η6-p-cymene)2}Fe{C5H 4P(OC6H3(OMe-o)(C3H 5-p))2}2] (12) in good yield. Treatment of 2 with copper chloride in a 1:1 or 1:2 molar ratio resulted in the formation of a binuclear complex, [{(CuCl)Fe{C5H4P(OC6H 3(OMe-o)(C3H5-p))2} 2}2] (13), whereas a similar reaction of 2 with CuBr and CuI in a 1:2 or 1:2.5 molar ratio yielded the novel butterfly-like deca-nuclear complexes [Cu5(μ-X)5{Fe{C5H 4P(OC6H3(OMe-o)(C3H 5-p))2}2}2]2 (14, X = Br; 15, X = I). The reaction of 2 with two equivalents of [AuCl(SMe2)] afforded the digold complex [(AuCl)2Fe{C5H 4P(OC6H3(OMe-o)(C3H 5-p))2}2] (16), with the ligand exhibiting bridged-bidentate mode of coordination. Additionally, some complexes were studied by cyclic voltammetry. The crystal structures of complexes 8, 9, 12, 15 and 16 were determined using X-ray diffraction studies.
Graphene oxide (GO) was functionalized with two organic ligands, triethylenetetramine (TETA) or 2,6-diaminopyridine (DAP), followed by palladium nanoparticles (Pd NPs) for the synthesis of Pd NPs/GO-TETA and Pd NPs/GO-DAP nanocomposites, respectively. The two heterogeneous nanocomposites were fully characterized and their efficiency was investigated for C[sbnd]C bond formation for the synthesis of biaryl compounds via the Suzuki cross-coupling reaction of aryl halides with arylboronic acid derivatives. The obtained results indicated that the Pd NPs/GO-TETA nanocomposite was more effective in the Suzuki coupling reaction as compared to Pd NPs/GO-DAP. Thus, the Suzuki cross-coupling reaction of different aryl halides with arylboronic acid derivatives using Pd NPs/GO-TETA nanocomposite catalyst in the presence of Na2CO3 as base in DMF/H2O (1/1) as solvent at 90 °C was carried out to afford the desired biaryl compounds in high to excellent yields (81–100%) and short reaction times (10–90 min). Additionally, Pd NPs/GO-TETA nanocomposite can be recovered and reused for 8 consecutive runs without any apparent loss of its catalytic activity, proving its high stability and potential use in organic transformations.
A protocol was described for obtaining a variety of substituted thiophenes with functional potential via metal-free dehydration and sulfur cyclization of alkynols with elemental sulfur (S8) or EtOCS2K in moderate-to-good yields. The method provides the base-free generation of a trisulfur radical anion (S3?-) and its addition to alkynes as an initiator. This research broadens the applications of S3?-in the synthesis of sulfur-containing heterocycles.
Development of safe, renewable, cheap and versatile solvents is a longstanding challenge in chemistry. We show here that vegetable oils and related systems can become prominent solvents for organic synthesis. Suzuki-Miyaura, Hiyama, Stille, Sonogashira and Heck cross-couplings proceed with quantitative yields in a range of vegetable oils, fish oil, butter and waxes used as solvents. Appropriate methodologies for high-throughput screening and sustainable isolation techniques applicable for vegetable oils and related lipids are presented.
Single atom catalysis has emerged as a powerful technique for catalysis due to its outstanding performance and atom economy. Controlling the hybridization of the atom with its environment is crucial in determining the selectivity and/or yield of the reaction. However, the single atom environment is usually ill-defined and hard to predict because the pyrolysis process used in preparing SACs damages the original status of the precursors in the catalyst preparation. A molecular engineering approach to synthesize single atom catalysts (SACs) on a heterogeneous template provides a strategy to make SACs with a highly uniform coordinating environment. Herein, we report the preparation of a molecular engineered Pd single atom catalyst with a pre-defined M-N3C1 coordination (Pd-N3C1-SAC) using a structure-rigid Pd-N3C1 porphyrin as the precursor, which shows more efficient Suzuki coupling compared with the SAC with Pd-N4 coordination. The origin of the high activity of the Pd-N3C1-SAC is revealed through density functional theory calculations, where a lower reaction barrier for the rate-determining oxidative addition is identified. This journal is
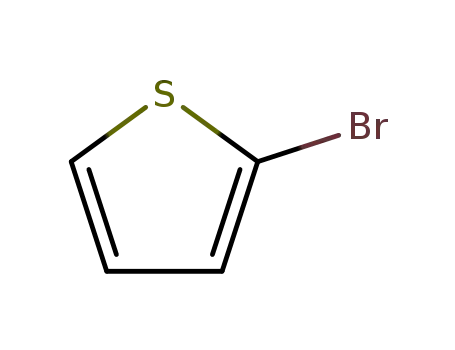
2-bromothiophene

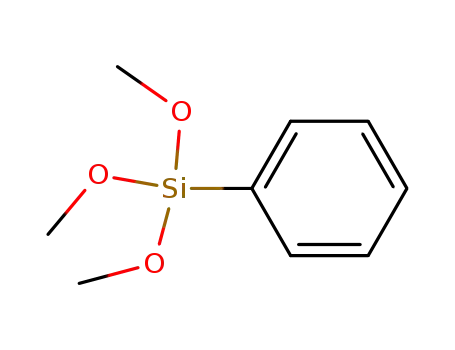
phenyl trimethylsiloxane

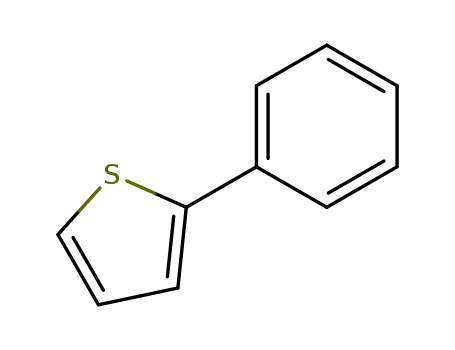
2-phenylthiophene

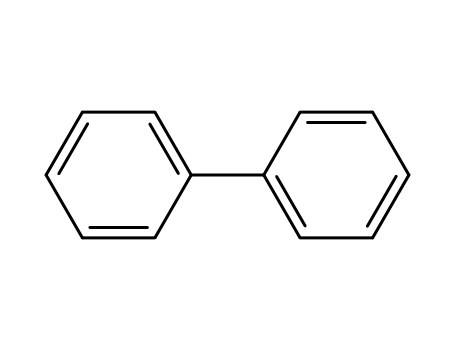
biphenyl

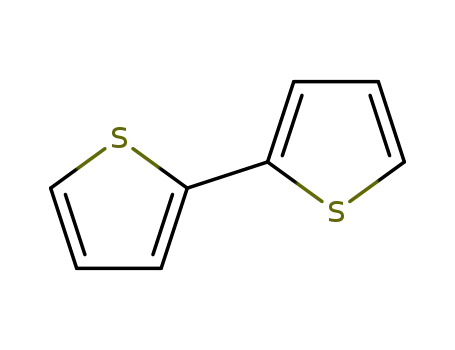
2,2'-Bithiophene
| Conditions | Yield |
|---|---|
|
With
tetrabutyl ammonium fluoride; triphenylphosphine;
palladium diacetate;
In
tetrahydrofuran; N,N-dimethyl-formamide;
at 90 ℃;
for 24h;
Title compound not separated from byproducts.;
|
30% |
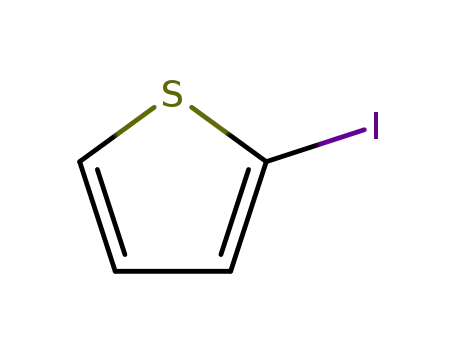
2-Iodothiophene

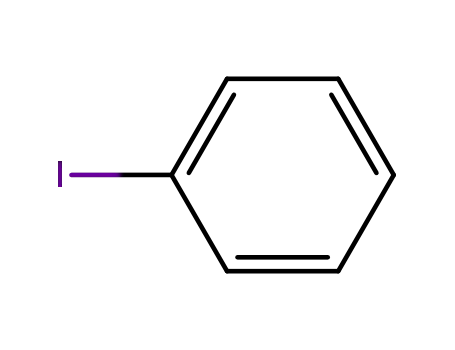
iodobenzene


2-phenylthiophene


biphenyl


2,2'-Bithiophene
| Conditions | Yield |
|---|---|
|
With
N-ethyl-N,N-diisopropylamine;
In
N,N-dimethyl-formamide;
at 140 ℃;
for 6h;
Overall yield = 81 percent;
Catalytic behavior;
|
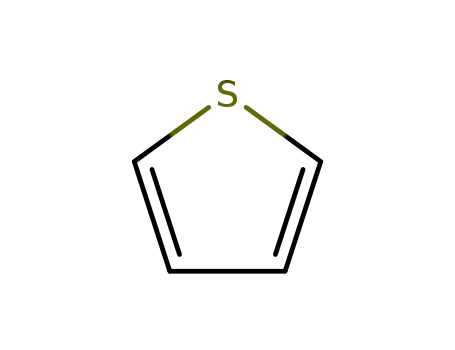
thiophene
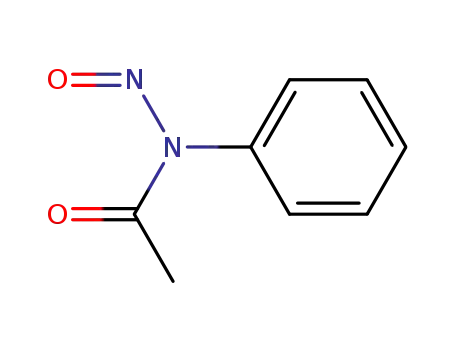
N-nitrosoacetanilide
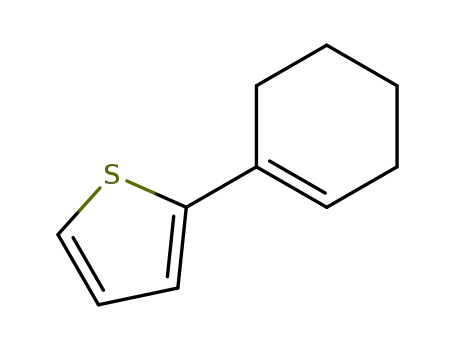
2-(cyclohex-1-en-1-yl)thiophene
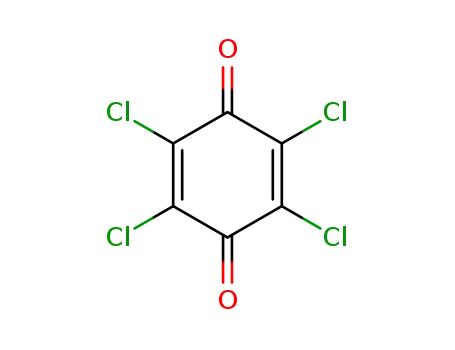
chloranil
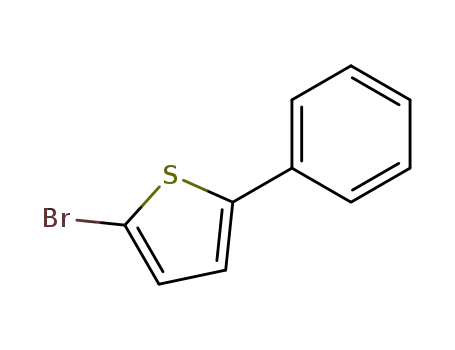
2-Bromo-5-phenyl-thiophene
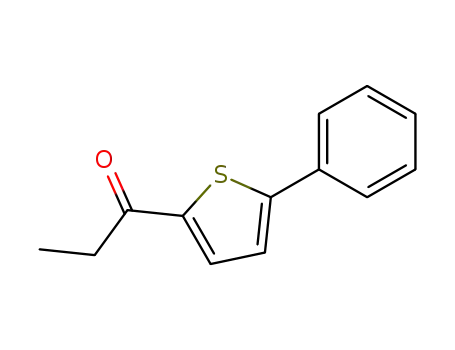
5-Phenyl-2-propionyl-thiophen
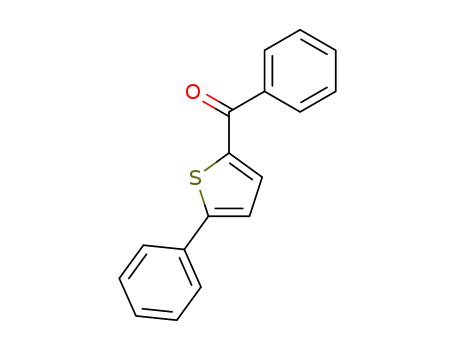
phenyl (5-phenylthiophen-2-yl) methanone
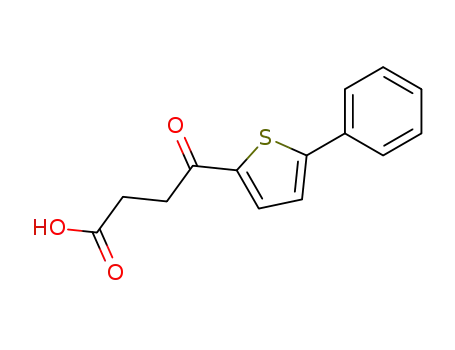
4-oxo-4-(5-phenyl-2-thienyl)butanoic acid
CAS:25550-51-0
Molecular Formula:C<sub>9</sub>H<sub>12</sub>O<sub>3</sub>
Molecular Weight:168.19
CAS:502161-03-7
CAS:34722-01-5
CAS:116971-11-0