Your Location:Home >Products >Organic phosphines >CyclohexyI phosphines >146960-90-9
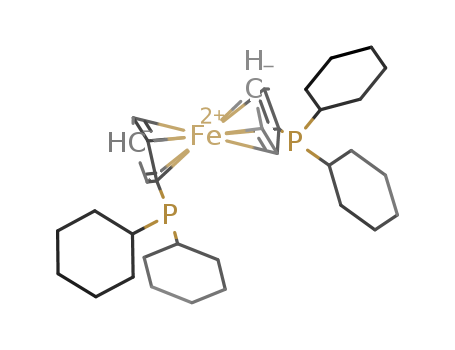

Product Details
Reaction
Ligand for palladium-catalyzed synthesis of oxindoles by amide ɑ-arylation. Ligand for palladium-catalyzed alkoxycarbonylation of aryl chlorides. Ligand for ruthenium-catalyzed alcohol-allene C-C coupling reaction via hydrohydroxyalkylation of 1,1-disubstituted allenes employing alcohols. Ligand for nickel-catalyzed cross-coupling reaction of arylboronic acids with aryl carbonates. Ligand for palladium-catalyzed regiodivergent hydroesterification of aryl olefins with phenyl formate to form linear structured phenyl arylpropanoates. Ligand for palladium-catalyzed direct borylation of benzyl alcohol and its analogues in the absence of bases.
The invention discloses a method for preparing ferrocene diphosphine ligand, and belongs to the field of organic synthesis. The method comprises the following steps: by taking ferrocene as an initial raw material and boron trifluoride diethyl etherate as a catalyst, reacting with diaryl phosphine oxide or dialkyl phosphine oxide, hydrolyzing so as to obtain tertfluoborate of a ferrocene diphosphine compound, and performing heating backflow deprotection in methanol, thereby obtaining the ferrocene diphosphine compound. Compared with the prior art, the method is gentle in reaction condition, simple in aftertreatment, and relatively applicable to industrial production, and the yield is greater than 90%. The prepared ferrocene diphosphine can be used as ligand of a metal catalyst, and can be used in the fields such as organic optoelectronic materials and medicines.
A computational screening of 42 bidentate phosphines (PP) has yielded promising candidates for Ph-CF3 reductive elimination from Ni(II) complexes of the type [(PP)Ni(Ph)(CF3)]. The computed barriers and synthetic accessibility consid
![[(1,1'-bis(dicyclohexylphosphino)ferrocene)Ni(1-naphthyl)Cl]](/upload/2023/2/a0e20042-c09d-4a05-a4e4-21c777b69fd5.png)
[(1,1'-bis(dicyclohexylphosphino)ferrocene)Ni(1-naphthyl)Cl]

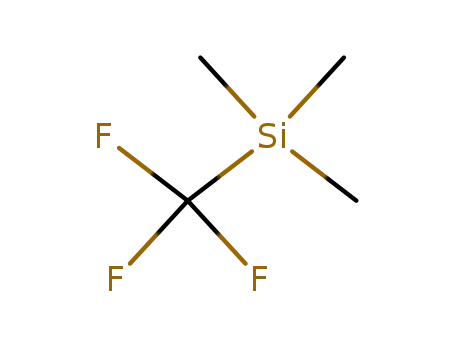
(trifluoromethyl)trimethylsilane


cesium fluoride

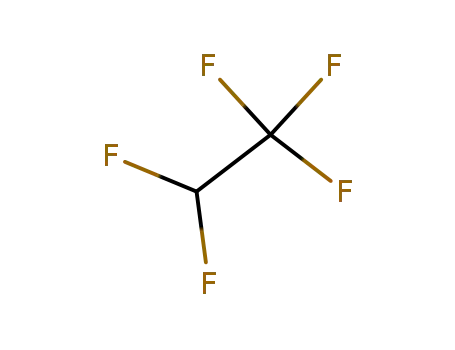
1,1,1,2,2-pentafluoroethane

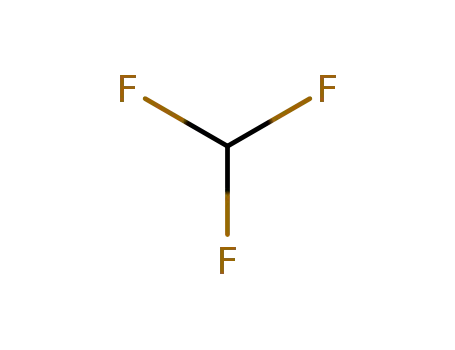
trifluoromethan

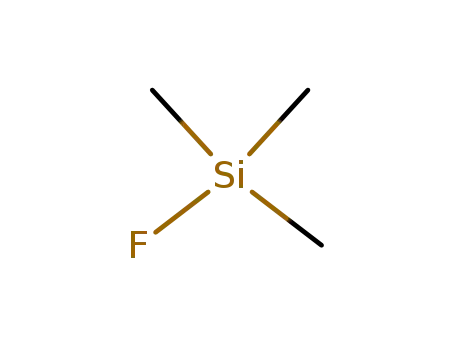
trimethylsilyl fluoride

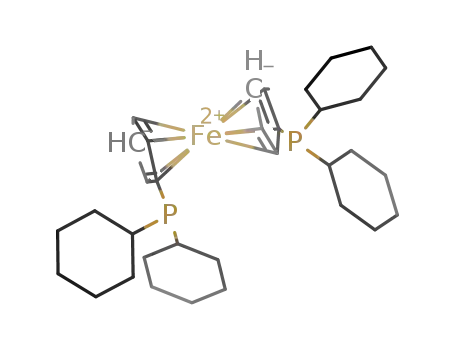
1,1'-bis(dicyclohexylphosphinocyclopentadienyl)iron

![[(1,1'-bis(dicyclohexylphosphino)ferrocene)Ni(1-naphthyl)F]](/upload/2023/2/012dcc1c-28d7-4c25-90bb-5345f7719865.png)
[(1,1'-bis(dicyclohexylphosphino)ferrocene)Ni(1-naphthyl)F]
| Conditions | Yield |
|---|---|
|
In
tetrahydrofuran;
at 20 ℃;
for 20h;
|
20 %Spectr. 2 %Spectr. Ca. 20 %Spectr. |
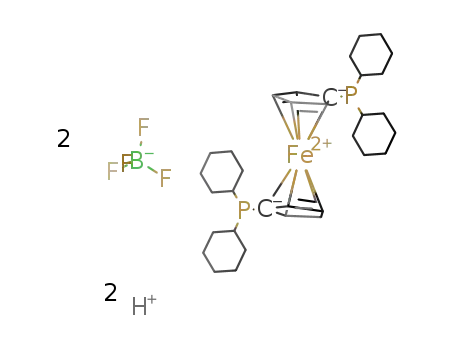
1,1'-bis(dicyclohexylphosphino)ferrocene tetrafluoroborate


1,1'-bis(dicyclohexylphosphinocyclopentadienyl)iron
| Conditions | Yield |
|---|---|
|
In
methanol;
for 12h;
Reflux;
|
92% |

(trifluoromethyl)trimethylsilane

cesium fluoride
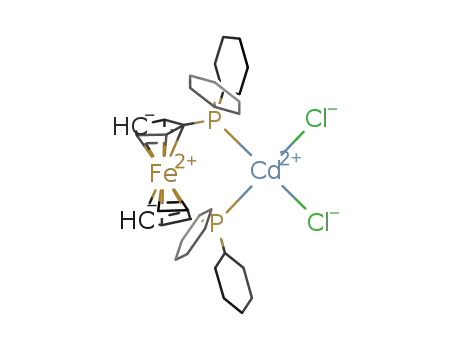
[CdCl2(1,1'-bis(dicyclohexylphosphino)ferrocene)]
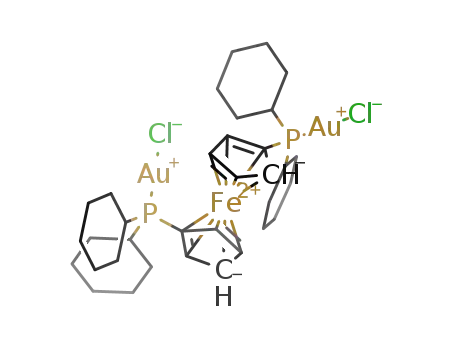
[Au2Cl2(1,1'-bis(dicyclohexylphosphino)ferrocene)]
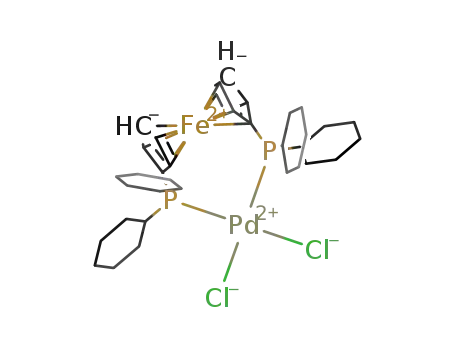
[1,1′-bis(di-cyclohexylphosphino)ferrocene]dichloropalladium (II)
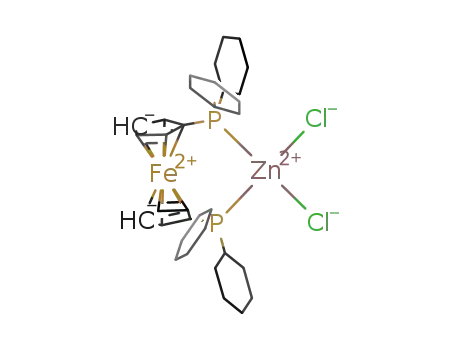
[ZnCl2(1,1'-bis(dicyclohexylphosphino)ferrocene)]
CAS:716-39-2
CAS:829-84-5