Your Location:Home >Products >Organic phosphines >CyclohexyI phosphines >564483-18-7

Product Details
|
Description |
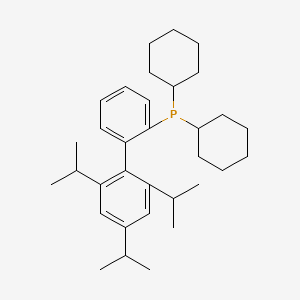
2-Dicyclohexylphosphino-2',4',6'-tri-i-propyl-1,1'-biphenyl is a phosphine ligand derived from biphenyl. Its palladium complexes exhibit high activity for Buchwald-Hartwig amination reactions involving aryl chlorides and aryl tosylates. Both palladium and copper complexes of the compound exhibit high activity for the coupling of aryl halides and aryl tosylates with various amides. It is also an efficient ligand for several commonly used C–C bond-forming cross-coupling reactions, including the Negishi, Suzuki, and the copper-free Sonogashira coupling reactions. It is especially efficient and general when employed as a (2-aminobiphenyl)-cyclometalated palladium mesylate precatalyst complex (Buchwald's third generation precatalyst system), XPhos-G3-Pd, which is commercially available and stable to bench storage. The ligand itself also has convenient handling characteristics as a crystalline, air-stable solid. |
|
Chemical Properties |
White crystalline powder |
|
Uses |
2-Dicyclohexylphosphino-2',4',6'-tri-i-propyl-1,1'-biphenyl may be used as a ligand in the following reactions: Preparation of functionalized benzylic sulfones via palladium-catalyzed Negishi cross-coupling between alkyl sulfones and aryl halides. 2-Dicyclohexylphosphino-2',4',6'-tri-i-propyl-1,1'-biphenyl may be used as a ligand in the following reactions: Preparation of functionalized benzylic sulfones via palladium-catalyzed Negishi cross-coupling between alkyl sulfones and aryl halides. Along with pre-milled palladium(II) acetate as a pre-catalyst for the Stille cross-coupling of aryl chlorides with tributylarylstannanes to form the corresponding biaryl compounds. Along with platinum chloride to catalyze the hydrosilylation of terminal arylalkynes with silanes to form functionalized β-(E)-vinylsilanes. |
Isomeric SMILES: CC(C)C1=CC(=C(C(=C1)C(C)C)C2=CC=CC=C2P(C3CCCCC3)C4CCCCC4)C(C)C
InChIKey: UGOMMVLRQDMAQQ-UHFFFAOYSA-N
InChI: InChI=1S/C33H49P/c1-23(2)26-21-30(24(3)4)33(31(22-26)25(5)6)29-19-13-14-20-32(29)34(27-15-9-7-10-16-27)28-17-11-8-12-18-28/h13-14,19-25,27-28H,7-12,15-18H2,1-6H3
Histochemical visualization of phosphatase is exclusively required for Western immunoblotting and antigen-positive cell staining using an alkaline phosphatase (AP)-labeled secondary antibody. This detection has been performed by several reagents including 5-bromo-4-chloro-3-indolyl-phosphate (X-Phos), nitro blue tetrazolium (NBT), 3-(2′-spiroadamantane)-4-methoxy-4-(3″-phosphoryloxy)phenyl-1,2-dioxetane and 2-(5′-chloro-2′-phosphoryloxyphenyl)-6-chloro-4-[3H]-quinazolinone (ELF® 97 Phosphate). We previously reported that 2-(benzothiazol-2-yl)-4-bromophenol bonded with N-acetylneuraminic acid (BTP3-Neu5Ac), enabled fluorescent histochemical visualization of sialidase activity.
The invention discloses a method for syn...
The invention discloses a method for pre...
Br versus Cl: It is found that the use o...
2,4,6-triisopropyl-1-bromobenzene

chlorodicyclohexylphosphane

2,3-dibromobenzene

XPhos
| Conditions | Yield |
|---|---|
|
2,4,6-triisopropyl-1-bromobenzene; With n-butyllithium; In tetrahydrofuran; at 0 ℃; for 1h; Inert atmosphere;
2,3-dibromobenzene; In tetrahydrofuran; at 0 ℃; for 1h; Inert atmosphere;
chlorodicyclohexylphosphane; Further stages;
|
89% |
fluorobenzene

1,3,5-triisopropyl benzene

chlorodicyclohexylphosphane

XPhos
| Conditions | Yield |
|---|---|
|
fluorobenzene; With lithium diisopropyl amide; In tetrahydrofuran; at -78 ℃; for 2h; Inert atmosphere;
chlorodicyclohexylphosphane; In tetrahydrofuran; at 15 ℃; for 10h;
1,3,5-triisopropyl benzene; With n-butyllithium; potassium tert-butylate; In hexane; at 30 - 60 ℃; for 16h; Reagent/catalyst; Temperature;
|
93% |
2-bromo-1-chlorobenzene
2,4,6-triisopropyl-1-bromobenzene
chlorodicyclohexylphosphane
dicyclohexylbromophosphine
tert-butyl 2-(benzamido)-4-(1H-indol-1-yl)benzoate
tert-butyl 2-(benzamido)-4-(2-nitroanilino)benzoate
tert-butyl 2-(benzamido)-4-(1H-pyrrol-1-yl)benzoate
tert-butyl 2-(benzamido)-4-(4-phenylpiperidin-1-yl)benzoate
CAS:98327-87-8
Molecular Formula:C<sub>44</sub>H<sub>32</sub>P<sub>2</sub>
Molecular Weight:622.7
CAS:787618-22-8
CAS:146960-90-9