Your Location:Home >Products >Organic phosphines >Other organic phosphines >98327-87-8

Product Details
|
Chemical Properties |
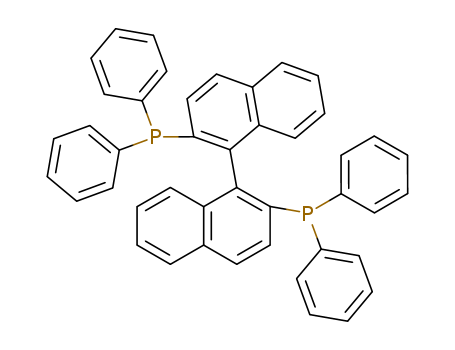
1.1'-Binaphthyl-2.2'-diphemyl phosphine is White to light beige powder |
|
Description |
(+/-)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl, also known as BINAP, is a chiral diphosphine ligand widely used in asymmetric catalysis. It is characterized by its axially dissymmetric bis(triaryl)phosphine structure, which imparts high enantioselectivity and reactivity in various organic reactions. |
|
Uses |
(+/-)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl (BINAP) is a chiral diphosphine ligand extensively used in asymmetric catalysis due to its high enantioselectivity and reactivity. It serves as a crucial ligand in various transition metal-catalyzed reactions, including hydrogenation, disilylation, and hydroformylation of olefins. BINAP, particularly in conjunction with rhodium and ruthenium, is a highly selective homogeneous catalyst for the reduction of aryl ketones, β-keto esters, and α-amino ketones, and is also used in asymmetric Heck reactions and isomerizations of allyls. Additionally, BINAP complexes with Ag(I) catalyze asymmetric aldol and hetero-Diels-Alder reactions. Its applications extend to palladium-catalyzed arylamine couplings, intramolecular acylations, and various C-C and C-N bond formations, demonstrating its versatility in organic synthesis. |
Reduction of phosphine oxides into the c...
Herein reported is the convenient and ef...
The invention relates to a synthesis met...
2,2’-bis(diphenylphosphinooxide)-1,1’-binaphthalene

2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl
| Conditions | Yield |
|---|---|
|
With trichlorosilane; triethyl phosphite; In tetrahydrofuran; toluene; at 100 ℃; for 3h;
|
95% |
|
With 1,3-diphenyl-disiloxane; In toluene; at 110 ℃; chemoselective reaction; Sealed tube;
|
94% |
|
2,2’-bis(diphenylphosphinooxide)-1,1’-binaphthalene; With 1,1,3,3-Tetramethyldisiloxane; titanium(IV) isopropylate; In toluene; at 85 ℃; for 20h;
With sodium hydroxide; water; In toluene; for 0.25h; Product distribution / selectivity;
|
91% |
|
2,2’-bis(diphenylphosphinooxide)-1,1’-binaphthalene; With trityl tetrakis(pentafluorophenyl)borate; In (2)H8-toluene; at 20 ℃; Glovebox; Inert atmosphere;
With phenylsilane; In (2)H8-toluene; at 100 ℃; for 24h; Glovebox; Inert atmosphere; Sealed tube;
|
23% |
|
With [AlH3(triethylamine)]; In hexane; at 20 ℃; for 0.166667h; Inert atmosphere; Schlenk technique;
|
96 %Chromat. |
2,2'-bis(diphenyloxyphosphino)-1,1'-binaphthyl

2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl
| Conditions | Yield |
|---|---|
|
With tributylphosphine; iodine; In tetrahydrofuran; acetonitrile; at 20 ℃; for 0.166667h; Inert atmosphere;
|
92% |
2,2'-dibromo-1,1'-binaphthyl
chloro-diphenylphosphine
diphenylarsane
(+/-)-2,2'-bis(trifluoromethanesulfonyloxy)-1,1-binaphthyl
(S)-(+)-1,1'-binaphthyl-2,2'-diylbis<(p-octylbenzyl)diphenylphosphonium> dibromide
2-(diphenylphosphinyl)-2'-(diphenylphosphanyl)-1,1'-binaphthalene
N-tert-butylaniline
N-(3,5-dimethylphenyl)-tert-butylamine
CAS:13716-12-6
Molecular Formula:C<sub>12</sub>H<sub>27</sub>P
Molecular Weight:202.32
CAS:1001911-63-2
Molecular Formula:C<sub>18</sub> H<sub>14</sub> BNO<sub>2</sub>
Molecular Weight:287.1
CAS:85-43-8
Molecular Formula:C<sub>8</sub>H<sub>8</sub>O<sub>3</sub>
Molecular Weight:152.15