Your Location:Home >Products >Acid Anhydrides >85-43-8

Product Details
|
Definition |
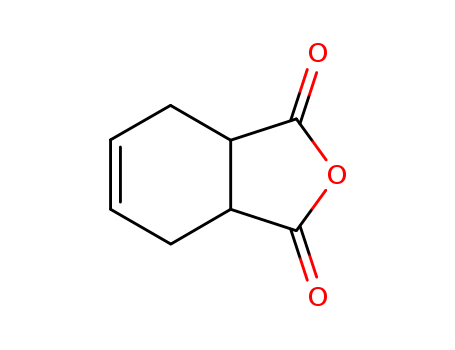
ChEBI: 1,2,3,6-tetrahydrophthalic anhydride is a cyclic dicarboxylic anhydride that is 1,3,3a,4,7,7a-hexahydro-2-benzofuran carrying oxo groups at positions 1 and 3. It is a cyclic dicarboxylic anhydride and a tetrahydrofurandione. |
|
General Description |
Clear colorless to light yellow slight viscous liquid. Highly toxic and a strong irritant to skin, eyes and mucous membranes. Corrosive to skin and metal. Used in making polyester, alkyd resins, and plasticizers. |
|
Air & Water Reactions |
Reacts exothermically with water to form memtetrahydrophthalic acid. |
|
Reactivity Profile |
CIS-1,2,3,6-TETRAHYDROPHTHALIC ANHYDRIDE; >98% is combustible. Reacts with oxidizers. Reacts exothermically with water. These reactions are usually slow, but can become violent when local heating accelerates their rate. Acids accelerate the reaction with water. Incompatible with acids, strong oxidizing agents, alcohols, amines, and bases. |
|
Health Hazard |
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution. |
|
Fire Hazard |
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. |
|
Flammability and Explosibility |
Nonflammable |
|
Safety Profile |
Moderately toxic by intraperitoneal route. Mildly toxic by ingestion. A corrosive irritant to skin, eyes, and mucous membranes. Combustible when exposed to heat or flame. Will react with water or steam to produce heat; can react with oxidizing materials. To fight fre, use water, foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes. See also ANHYDRIDES. |
InChI:InChI=1/C8H8O3/c9-7-5-3-1-2-4-6(5)8(10)11-7/h1,3,5-6H,2,4H2
The invention discloses a method for pre...
Cyclic anhydrides, key intermediates of ...
Solvent-free Diels-Alder reactions were ...
A catalyst for alkylation contains an in...
o-xylene

dimethyl cyclohex-4-ene-1,2-dicarboxylate

3,4-dimethoxycarbonylcyclohexyl-3′,4′-dimethylbenzene

1,2,3,6-Tetrahydrophthalic anhydride
| Conditions | Yield |
|---|---|
|
With Tridecane; In methanol; at 180 ℃; for 2h; Reagent/catalyst; Concentration; Inert atmosphere;
|
92.2% |
maleic anhydride

buta-1,3-diene

1,2,3,6-Tetrahydrophthalic anhydride
| Conditions | Yield |
|---|---|
|
at 80 - 110 ℃; for 4h; Temperature; Large scale;
|
98.1% |
|
|
80% |
|
With benzene; at 100 ℃;
|
|
|
With benzene; at 115 - 145 ℃;
|
|
|
With benzene; at 20 ℃;
|
|
|
In 1,4-dioxane; at 30 ℃; Yield given;
|
|
|
In 1,4-dioxane; at 30 ℃; Rate constant; Kinetics;
|
|
|
|
|
|
|
|
|
In neat (no solvent); at 30 ℃; for 2h; Green chemistry;
|
95.6 %Chromat. |
maleic anhydride
2,3-dimethylbutene
buta-1,3-diene
3-Sulfolene
6β-((1Ξ)-ξ-6-carboxy-cyclohex-3-enecarbonylamino)-penicillanic acid
6-thiazol-2-ylcarbamoyl-cyclohex-3-enecarboxylic acid
1,2,3,6-Tetrahydrophthalsaeure-bis-dimethylamid
trimethylsilyl cis-2-isocyanato-4-cyclohex-1-ene carboxylate
CAS:120-74-1
Molecular Formula:C<sub>8</sub>H<sub>10</sub>O<sub>2</sub>
Molecular Weight:138.16
CAS:13716-12-6
Molecular Formula:C<sub>12</sub>H<sub>27</sub>P
Molecular Weight:202.32