Your Location:Home >Products >OLED intermediates >Boric acids >1001911-63-2

Product Details
|
Uses |
(9-phenyl-9H-carbazol-2-yl)boronic acid can be used as an intermediate in organic synthesis, mainly used in laboratory research and development and in the process of chemical and pharmaceutical synthesis. |
|
Synthesis |
Sub 1-3-1 (6.4g, 20mmol) was dissolved in anhydrous Ether, lowering the temperature of the reaction to -78°C, n-BuLi (2.5M in hexane) (1.4g, 22mmol) was added dropwise slowly, and after, the reaction It was stirred for 30 minutes. After lowering the temperature of the reaction back to -78°C dropwise Triisopropyl borate (5.6g, 30mmol). Stirring at room temperature, diluted with water and it binds the 2N HCl. After completion of reaction, ethyl acetate and water, dried over MgSO4 and the organic layer was extracted and recrystallized silicagel column and the resulting organic material and then concentrated to a Sub 1-4-1 4.5g (yield: 78%) was obtained. |
With respect to the commonly used electr...
The present invention relates to an orga...
The present invention relates to a compo...
4-Bromophenylboronic acid

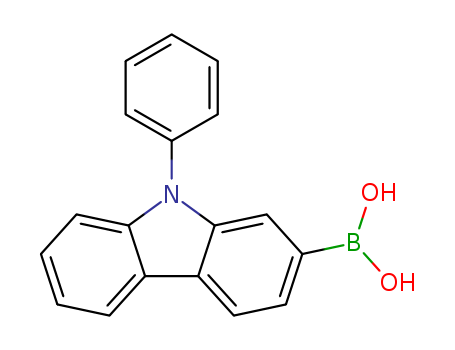
(9‐phenyl‐9H‐carbazol‐2‐yl)boronic acid
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 4 steps
1.1: sodium carbonate / tetrakis(triphenylphosphine) palladium(0) / toluene / 4 h / 100 °C
2.1: triethyl phosphite / 6 h / 150 °C
3.1: caesium carbonate / copper(l) iodide / toluene / 50 °C
3.2: 14 h / Reflux
4.1: n-butyllithium / hexane; tetrahydrofuran / 1 h / -78 °C
4.2: 12 h / 20 °C
With n-butyllithium; sodium carbonate; caesium carbonate; triethyl phosphite; copper(l) iodide; tetrakis(triphenylphosphine) palladium(0); In tetrahydrofuran; hexane; toluene;
|
|
|
Multi-step reaction with 4 steps
1.1: sodium carbonate / tetrakis(triphenylphosphine) palladium(0) / toluene / 4 h / 100 °C
2.1: triethyl phosphite / 6 h / 150 °C
3.1: caesium carbonate / copper(l) iodide / toluene / 50 °C
3.2: 14 h / Reflux
4.1: n-butyllithium / hexane; tetrahydrofuran / 1 h / -78 °C
4.2: 12 h
With n-butyllithium; sodium carbonate; caesium carbonate; triethyl phosphite; copper(l) iodide; tetrakis(triphenylphosphine) palladium(0); In tetrahydrofuran; hexane; toluene;
|
4'-bromo-2-nitrobiphenyl

(9‐phenyl‐9H‐carbazol‐2‐yl)boronic acid
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1.1: triethyl phosphite / 6 h / 150 °C
2.1: caesium carbonate / copper(l) iodide / toluene / 50 °C
2.2: 14 h / Reflux
3.1: n-butyllithium / hexane; tetrahydrofuran / 1 h / -78 °C
3.2: 12 h / 20 °C
With n-butyllithium; caesium carbonate; triethyl phosphite; copper(l) iodide; In tetrahydrofuran; hexane; toluene;
|
|
|
Multi-step reaction with 3 steps
1.1: triethyl phosphite / 6 h / 150 °C
2.1: caesium carbonate / copper(l) iodide / toluene / 50 °C
2.2: 14 h / Reflux
3.1: n-butyllithium / hexane; tetrahydrofuran / 1 h / -78 °C
3.2: 12 h
With n-butyllithium; caesium carbonate; triethyl phosphite; copper(l) iodide; In tetrahydrofuran; hexane; toluene;
|
|
|
Multi-step reaction with 3 steps
1.1: triethyl phosphate / 7 h / 150 °C
2.1: potassium phosphate; ethylenediamine / 12 h / Reflux
3.1: n-butyllithium / tetrahydrofuran; hexane / 1 h / -78 °C
3.2: 12 h / -78 - 20 °C
3.3: 20 °C
With potassium phosphate; n-butyllithium; triethyl phosphate; ethylenediamine; In tetrahydrofuran; hexane;
|
4-Bromophenylboronic acid
4'-bromo-2-nitrobiphenyl
2-bromo-9H-carbazole
o-nitroiodobenzene
2-(2-nitrophenyl)-9-phenyl-9H-carbazole
5-phenyl-5,11-dihydro-indolo[3,2-b]carbazole
C30H19BrN2
C30H21BN2O2
CAS:1219956-23-6
Molecular Formula:C27H26BN3O2
Molecular Weight:435.3
CAS:28320-31-2
Molecular Formula:C<sub>15</sub>H<sub>13</sub>Br
Molecular Weight:273.17
CAS:98327-87-8
Molecular Formula:C<sub>44</sub>H<sub>32</sub>P<sub>2</sub>
Molecular Weight:622.7