Your Location:Home >Products >Functional intermediates >1219956-23-6

Product Details
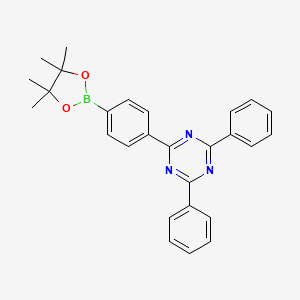
| Description | 2,4-Diphenyl-6-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]-1,3,5-triazine is an organic compound containing a 1,3,5-triazine core with two phenyl groups and one 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl group. The boron-containing substituent allows for facile coupling reactions, making this molecule a useful building block in organic synthesis, particularly in the preparation of functionalized heterocycles and materials. This compound has found applications in areas such as organic electronics, photovoltaics, and bioimaging due to its interesting photophysical properties and ability to undergo various derivatization reactions. |
| Purity and Storage | Purity: Minimum 98% (GC) Storage Temperature: Ambient |
| Chemical Structure | Comprises 2,4-Diphenyl-6-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]-1,3,5-triazine. |
| Functional Groups | Contains phenyl rings, a triazine ring, and a reactive boronic acid group. Boronic acid groups are known for their reactivity in certain chemical reactions, such as Suzuki-Miyaura coupling. |
| Uses | Compounds with boronic acid functionalities are commonly used in organic synthesis, especially in Suzuki-Miyaura coupling reactions. These reactions are instrumental in constructing biaryl compounds. |
InChI:InChI=1/C10H9BO2/c12-11(13)10-7-3-5-8-4-1-2-6-9(8)10/h1-7,12-13H
Mix 2-(2-bromophenyl)-9-phenyl-9H-carbazole (1 g, 2.51 mmol), 2,4-diphenyl-6-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)-1,3,5-triazine (1.2 g, 2.76 mmol), K2CO3 (0.7 g, 5.06 mmol) and Pd(PPh3)2Cl2 (0.02 g, 0.03 mmol) in a three-necked flask, followed by toluene (10 mL), ethanol (5 mL) and deionized water (5 mL).
The present specification provides a com...
1.4-dibromobenzene
p-bromophenylboronic acid pinacol ester
CAS:1001911-63-2
Molecular Formula:C<sub>18</sub> H<sub>14</sub> BNO<sub>2</sub>
Molecular Weight:287.1
CAS:23449-08-3
CAS:90-41-5