Your Location:Home >Products >Functional intermediates >23449-08-3

Product Details
Chemical Properties
white solid powder
A new donor moiety, 7,7,13,13-tetramethyl-7,13-dihydro-5H-indeno[1,2-b]acridine (IAc), was developed to control the highest occupied molecular orbital (HOMO) dispersion of thermally activated delayed fluorescent (TADF) emitters. The IAc unit expanded the HOMO dispersion of the emitters and increased the quantum efficiency of the TADF devices up to 20.9 %.
The invention belongs to the technical field of organic materials, and provides a nitrogen-containing compound, and an organic electroluminescent device and an electronic device using the same. The nitrogen-containing compound has a structure as shown in a formula I, wherein X1, X2 and X3 are respectively and independently selected from N or CH, and at least one of X1, X2 and X3 is N. When the nitrogen-containing compound is used in the organic electroluminescent device, the performance of the device can be improved.
The present application provides a compound of formula (I), which may be used in an electron transport material. The compound has the parent structure of electrosorption fragment biphenanthroline, has high bond energy among atoms, has good thermal stability, is favorable for solid accumulation of molecules, has strong electronic transition capability, can effectively reduce the driving voltage of an organic electroluminescent device, improves the current efficiency and prolongs the service life. The invention further provides an organic electroluminescent device comprising the compound of the general formula (I) and a display device.
The present invention relates to an organic light emitting compound represented by chemical formula 1 and an organic electroluminescent device including the same, and the organic light emitting compound according to the present invention has excellent luminous efficiency and material lifetime properties, and thus, the organic electroluminescent device having excellent luminous efficiency while having power efficiency and long durability can be manufactured. Chemical formula 1.
The invention relates to the technical field of organic photoelectric materials, and in particular, relates to an organic compound, an electronic element containing the same and an electronic device.The compound has a structure represented by a chemical formula 1', wherein one of R1, R2, R3 and R4 is a group defined in the specification, and the other three are selected from substituents such asalkyl, halogen and cyano; one of R5, R6, R7 and R8 is a group defined in the specification, the other three are selected from substituents such as alkyl, halogen and cyano, Y and Y1 are respectively and independently a group defined in the specification, and L and L1 are single bonds, aryl, heteroaryl and the like. By using the organic compound in an electronic component, the driving voltage, luminous efficiency, and life of the electronic component are improved.
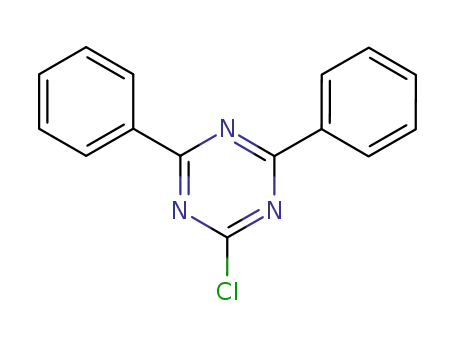
2-chloro-4,6-diphenyl-1,3,5-triazine

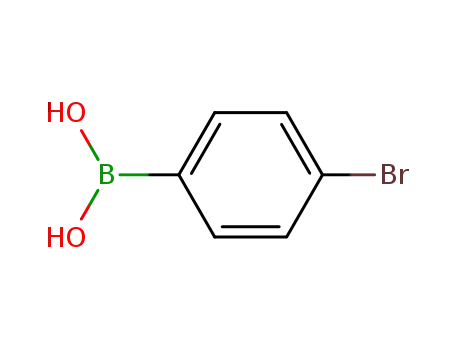
4-Bromophenylboronic acid

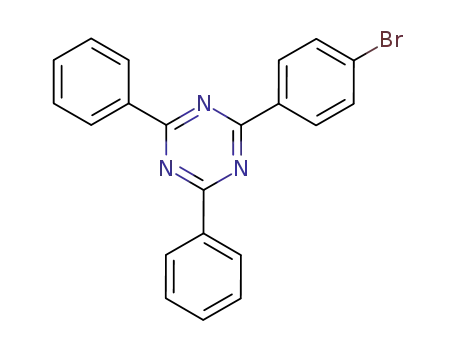
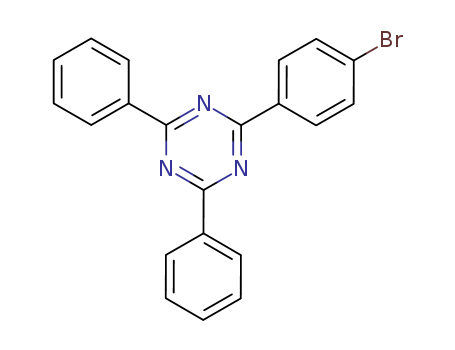
2-(4-bromobenzenyl)-4,6-diphenyl-1,3,5-triazine
| Conditions | Yield |
|---|---|
|
With
tetrakis(triphenylphosphine) palladium(0); potassium carbonate;
In
tetrahydrofuran; water;
at 80 ℃;
for 12h;
Inert atmosphere;
|
96% |
|
With
tetrakis(triphenylphosphine) palladium(0); potassium carbonate;
In
tetrahydrofuran; water; toluene;
for 12h;
Reflux;
|
96.5% |
|
With
tetrakis(triphenylphosphine) palladium(0); tetrabutyl-ammonium chloride; potassium carbonate;
In
ethanol; water; toluene;
at 78 ℃;
for 10h;
Inert atmosphere;
|
82% |
|
With
tetrakis(triphenylphosphine) palladium(0); potassium carbonate;
In
1,4-dioxane; water; toluene;
at 100 ℃;
for 12h;
|
80% |
|
With
tetrakis(triphenylphosphine) palladium(0); tetrabutyl-ammonium chloride; potassium carbonate;
In
ethanol; water; toluene;
at 78 ℃;
for 8h;
Inert atmosphere;
|
80% |
|
With
tetrakis(triphenylphosphine) palladium(0); tetrabutyl-ammonium chloride; potassium carbonate;
In
ethanol; water; toluene;
at 78 ℃;
for 8h;
Inert atmosphere;
|
80% |
|
With
tetrakis(triphenylphosphine) palladium(0); potassium carbonate;
In
tetrahydrofuran; water;
at 80 ℃;
for 10h;
Inert atmosphere;
|
75.4% |
|
2-chloro-4,6-diphenyl-1,3,5-triazine; 4-Bromophenylboronic acid;
With
tetrakis(triphenylphosphine) palladium(0);
In
tetrahydrofuran;
for 0.5h;
Inert atmosphere;
With
potassium carbonate;
In
tetrahydrofuran; water;
at 80 ℃;
Inert atmosphere;
|
69% |
|
With
tetrakis(triphenylphosphine) palladium(0); potassium carbonate;
In
ethanol; water; toluene;
at 100 ℃;
for 12h;
|
60% |
|
With
tetrakis(triphenylphosphine) palladium(0); potassium carbonate;
In
tetrahydrofuran; water;
at 80 ℃;
for 53h;
Inert atmosphere;
|
60% |
|
With
tetrakis(triphenylphosphine) palladium(0); potassium carbonate;
In
tetrahydrofuran;
for 3h;
Reflux;
|
51% |
|
With
tetrakis(triphenylphosphine) palladium(0); potassium carbonate;
In
1,4-dioxane; water;
at 60 ℃;
for 18h;
Inert atmosphere;
|
30% |
|
With
tetrakis(triphenylphosphine) palladium(0); tetrabutylammomium bromide; potassium carbonate;
In
ethanol; water; toluene;
at 40 ℃;
for 5h;
Inert atmosphere;
Reflux;
|
30% |
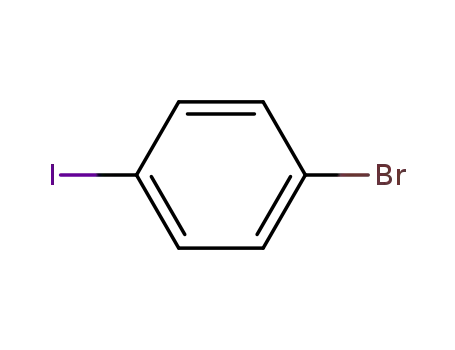
1,4-bromoiodobenzene

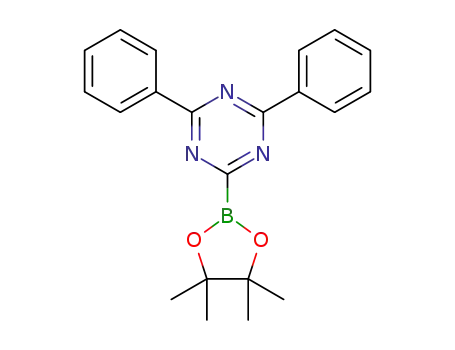
2,4-diphenyl-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolane-2-yl)-1,3,5-triazine


2-(4-bromobenzenyl)-4,6-diphenyl-1,3,5-triazine
| Conditions | Yield |
|---|---|
|
With
tetrakis(triphenylphosphine) palladium(0); potassium carbonate;
In
tetrahydrofuran; water;
for 10h;
Reflux;
Inert atmosphere;
|
83% |
|
With
tetrakis(triphenylphosphine) palladium(0); sodium carbonate;
In
water; toluene;
for 8h;
Inert atmosphere;
Reflux;
|
82% |
|
With
tetrakis(triphenylphosphine) palladium(0); sodium carbonate;
In
water; toluene;
for 8h;
Inert atmosphere;
Reflux;
|
82% |
|
With
tetrakis(triphenylphosphine) palladium(0); potassium carbonate;
In
water; toluene;
at 70 ℃;
for 11h;
Inert atmosphere;
|
80.44% |
|
With
tetrakis(triphenylphosphine) palladium(0); potassium carbonate;
In
water; toluene;
at 70 ℃;
for 11h;
Inert atmosphere;
|
80.44% |
|
With
tetrakis(triphenylphosphine) palladium(0); potassium carbonate;
In
water; toluene;
at 70 ℃;
for 11h;
Inert atmosphere;
|
80.44% |
|
With
tetrakis(triphenylphosphine) palladium(0); tetrabutylammomium bromide; potassium carbonate;
In
ethanol; water; toluene;
at 72 ℃;
for 10h;
Inert atmosphere;
|
80% |

2-chloro-4,6-diphenyl-1,3,5-triazine
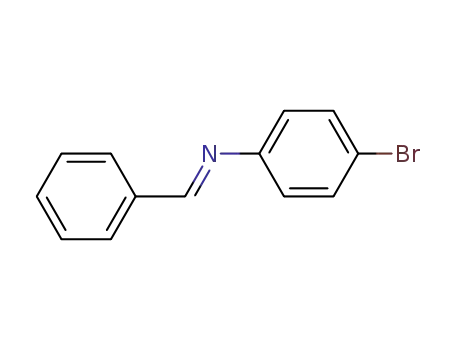
4-bromo-N-[(E)-phenylmethylidene]aniline
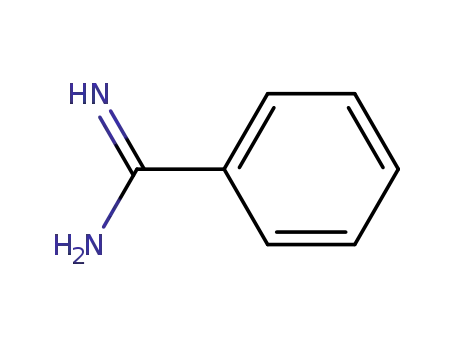
benzamidin
3-allyl-6-(9-(2-methylpropyl)carbazol-3-yl)-9-(2-methylpropyl)carbazole
2,4-diphenyl-6-(4'-triphenylsilanylbiphenyl-4-yl)-1,3,5-triazine
C51H33N5
2,4-diphenyl-6-(4-(4,4',5,5'-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)-1,3,5-triazine
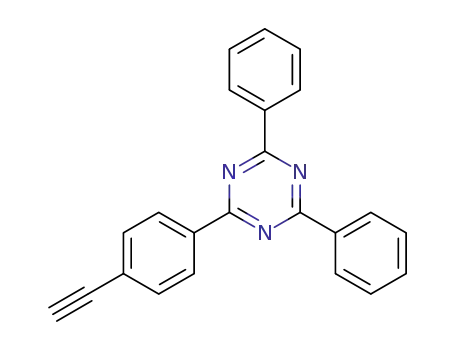
2-(4-ethynylphenyl)-4,6-diphenyl-1,3,5-triazine
CAS:502161-03-7
CAS:161265-03-8
Molecular Formula:C39H32OP2
Molecular Weight:578.6
CAS:864377-31-1
CAS:1219956-23-6
Molecular Formula:C27H26BN3O2
Molecular Weight:435.3