Your Location:Home >Products >Functional intermediates >90-41-5

Product Details
Description
2-Aminobiphenyl (2-APB) is an organic compound with the formula C6H5C6H4NH2. It is an amine derivative of biphenyl. It is a colorless solid, although aged samples can appear colored even black. Palladacycles obtained from 2-aminobiphenyl are popular catalysts for cross-coupling.
Chemical Properties
colourless to purple crystalline solid. Soluble in alcohol, ether and benzene, insoluble in water. Can be volatilized with water vapor. Flash point >110℃.
Uses
2-Aminobiphenyl has a mutagenic potency. It is a substrate for UGTs (UDP-glucuronosyltransferases), including UGT1A4, UGT2B13 and UGT2B16.
Preparation
2-Aminodiphenyl is prepared by hydrogenation of 2-nitrobiphenyl.NEW SYNTHESIS OF 2-AMINOBIPHENYLSReflux 2-nitrobiphenyl, ethanol and stannous chloride in a water bath for 3h, recover the ethanol, cool the residue, add 40% liquid alkali to make it strongly alkaline, separate the oil layer, extract the aqueous layer with ether, dry the extract with anhydrous calcium chloride, filter and recover the ether, wash with ethanol to obtain the crude product, then distill the crude product under reduced pressure to obtain 2-aminobiphenyl.
Application
2-Aminobiphenyl is used as raw materials for organic synthesis. manufacture of carbazole resin, synthetic rubber.
Synthesis Reference(s)
Organic Syntheses, Coll. Vol. 5, p. 829, 1973Tetrahedron Letters, 36, p. 125, 1995 DOI: 10.1016/0040-4039(94)02191-D
General Description
2-aminobiphenyl appears as colorless or purplish crystals. (NTP, 1992)
Air & Water Reactions
Insoluble in water.
Reactivity Profile
2-Aminodiphenyl neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. May generate hydrogen, a flammable gas, in combination with strong reducing agents such as hydrides.
Hazard
Toxic by ingestion, inhalation, and skin absorption. A carcinogen.
Fire Hazard
2-Aminodiphenyl is probably combustible.
Flammability and Explosibility
Nonflammable
Safety Profile
Moderately toxic by ingestion.Mutation data reported. When heated to decomposition, itemits toxic fumes of NOx.
Purification Methods
Crystallise it from aqueous EtOH (charcoal). [Beilstein 12 IV 3223.]
InChI:InChI=1/C12H11N/c13-12-9-5-4-8-11(12)10-6-2-1-3-7-10/h1-9H,13H2
RhCl3 was heterogenized into renewable polysaccharide supports, and the effect of polysaccharide type on the new catalyst performances in a Suzuki cross-coupling reaction was studied. The conversion of the fresh iota carrageenan heterogeneous system (?-RhCl3), was found to be the highest, whereas it was lower than the conversion of its homogeneous analogue. In addition, the ?-RhCl3 (catalyst loading of 6.5 % wt) was proven to be efficient heterogeneous catalyst that was easily recycled, whereas the conversion was increased in the first and second cycles. Scanning electronic microscopy (SEM) combining Energy dispersive X-ray spectrometry and Surface analysis by X-ray photoelectron spectroscopy were performed, confirming that RhCl3 was embedded within the ?-carrageenan. The Fourier-transform infrared spectrometry of the heterogeneous ?-RhCl3 catalyst was compared to that of the native polysaccharide, and no new bands were detected. Nonetheless, a comparison of SEM image of ?-carrageenan with and without RhCl3, as well as rheological measurements of the aqueous solution of ? with and without RhCl3, indicated the incorporation of the polysaccharide with the RhCl3.
A simple and green protocol for the synthesis of poly(ethylene glycol) stabilized palladium nanoparticles under ambient conditions from the aqueous extracts of Colocasia esculenta leaves has been reported. The nanoparticles are characterized using UV-visible spectroscopy, FTIR spectroscopy, XRD and SEM analysis. The prepared Pd NPs showed excellent catalytic activity towards Suzuki-Miyaura cross coupling reactions for a wide variety of aryl halides and phenyl boronic acid substrates. The catalytic system was found to be recyclable and could be reused in subsequent catalytic runs without significant loss of activity.
The nanoreactor of hollow magnetic mesoporous silica spheres (Pd/HMMS), with Pd and Fe3O4 nanoparticles embedded in the mesoporous silica shell, were successfully prepared by using the colloidal carbon spheres of glucose, Pd and Fe3O4 heteroaggregates as the hard template together with a coating of tetraethoxysilane (TEOS) and cetyltrimethylammonium bromide (CTAB) mixture. The synthesized Pd/HMMS shows excellent catalytic activity in the Suzuki cross-coupling reaction of iodobenzene with phenylboronic acid with over 99% yield in 3 min and can be recycled multiple times without any significant loss in catalytic activity.
Well distributed Pd-Cu bimetallic alloy nanoparticles supported on amine-terminated ionic liquid functional three-dimensional graphene (3D IL-rGO/Pd-Cu) as an efficient catalyst for Suzuki cross-coupling reaction has been prepared via a facile synthetic method. The introduction of IL-NH2 cations on the surface of graphene sheets can effectively avoid the re-deposition of graphene sheets, allowing the catalyst to be reused up to 10?cycles. The addition of Cu not only saves cost but also ensures high catalytic efficiency. It is worthy to note that the catalyst 3D IL-rGO/Pd2.5Cu2.5 can efficiently catalyze the Suzuki cross-coupling reaction with the yield up to 100% in 0.25?h, almost one-fold higher than that by the pristine IL-rGO/Pd2.5 catalyst (52%). The Powder X-Ray Diffraction (XRD), combining energy dispersive X-ray spectroscopy (EDS) mapping results confirm the existence and distribution of Pd and Cu in the bimetallic nanoparticles. The transmission electron microscopy (TEM) reveals the nanoparticle size with an average diameter of 3.0?±?0.5?nm. X-ray photoelectron spectroscopy (XPS) analysis proved the presence of electron transfer from Cu to Pd upon alloying. Such alloying-induced electronic modification of Pd-Cu alloy and 3D ionic liquid functional graphene with large specific surface area both accounted for the catalytic enhancement.
A molecular probe for the luminescent detection of adenosine nucleotides is presented. The probe, Tb-DOTAm-Phen, readily distinguishes among the three adenosine nucleotides in buffered aqueous conditions at neutral pH, a requirement for the direct monitoring of enzymatic reactions converting adenosine triphosphate (ATP) to adenosine diphosphate or adenosine monophosphate. The probe is most efficient under millimolar concentrations of ATP which are relevant to intracellular conditions. Moreover, the long luminescence lifetime of the probe readily enables time-gating experiments.
Hydrodehalogenation is an effective strategy for transforming persistent and potentially toxic organohalides into their more benign congeners. Common methods utilize Pd/C or Raney-nickel as catalysts, which are either expensive or have safety concerns. In this study, a nickel-based catalyst supported on titania (Ni-phen@TiO2-800) is used as a safe alternative to pyrophoric Raney-nickel. The catalyst is prepared in a straightforward fashion by deposition of nickel(II)/1,10-phenanthroline on titania, followed by pyrolysis. The catalytic material, which was characterized by SEM, TEM, XRD, and XPS, consists of nickel nanoparticles covered with N-doped carbon layers. By using design of experiments (DoE), this nanostructured catalyst is found to be proficient for the facile and selective hydrodehalogenation of a diverse range of substrates bearing C?I, C?Br, or C?Cl bonds (>30 examples). The practicality of this catalyst system is demonstrated by the dehalogenation of environmentally hazardous and polyhalogenated substrates atrazine, tetrabromobisphenol A, tetrachlorobenzene, and a polybrominated diphenyl ether (PBDE).
The invention discloses a triptycene cyclometalated palladium compound and an application thereof. The triptycene cyclometalated palladium compound has the following general formula, wherein three RAscan be the same or different and are respectively and independently expressed as R1-(Z1-A1-Z2)x-,wherein the three RBs can be the same or different and are respectively and independently expressed asR2-(Z3-A2-Z4)y-, the two RCs may be the same or different, each independently expressed as R3-(Z5-A3-Z6)z-. The triptycene cyclometalated palladium compound provided by the invention has a triptycenelarge-steric-hindrance group, and can stabilize a zero-valent palladium intermediate in a catalytic cycle, so that the catalytic efficiency is improved, the use amount of a catalyst can be reduced tobe less than ten thousandth, and the compound is simple in synthesis step, high in yield, relatively low in cost and suitable for various substrates, and the method has an important application valuefor researching the progress and application of the coupling reaction.
Starting from cyclic diaryliodoniums and terminal alkenes, a diverse set of fluorenes is conveniently constructed. The reactions catalyzed by palladium undergo one conventional Mizoroki-Heck reaction and one reductive Heck reaction. The scope of alkenes is general, leading to 29 fluorenes which would expand the structural diversity of fluorene reservoir. (Figure presented.).
Abstract: Several highly efficient and magnetically recyclable cobalt catalytic systems were prepared using magnetic chitosan and some safe and available organic compounds (Co-ligand@MNPs/Ch). The structure of these nanocomposites was confirmed by various physicochemical techniques such as FT-IR, XRD, TGA, VSM, TEM, SEM, CHNS and ICP-OES. These nano composites exhibit remarkable catalytic efficiency for Suzuki and Heck cross-coupling reactions in mild and green reaction conditions. The facile accessibility of starting materials, possible performance in air and eco-friendly conditions are merits of our catalysts. In addition, to describe and go insight to role and effect of ligands present in these catalysts, electrostatic interactions, density functional theory (DFT) model in molecular method were employed. Graphic Abstract: [Figure not available: see fulltext.]
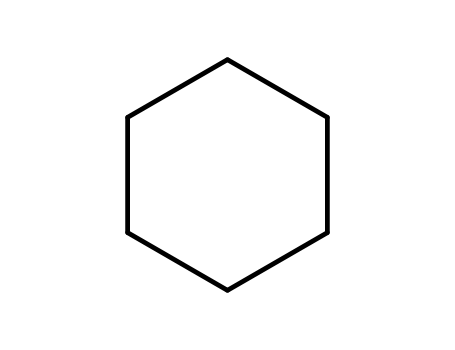
cyclohexane

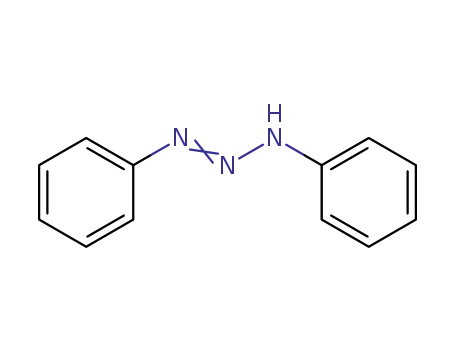
1,3-Diphenyltriazen

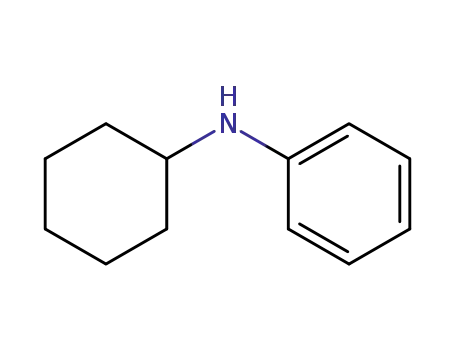
N-phenyl-2-cyclohexylamine

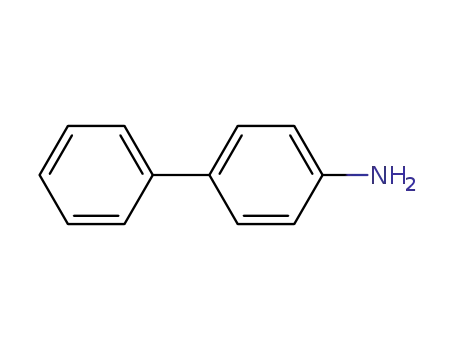
4-phenylalanine

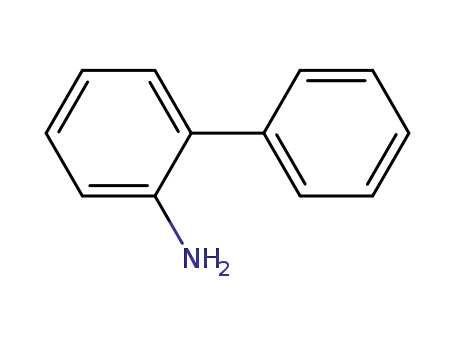
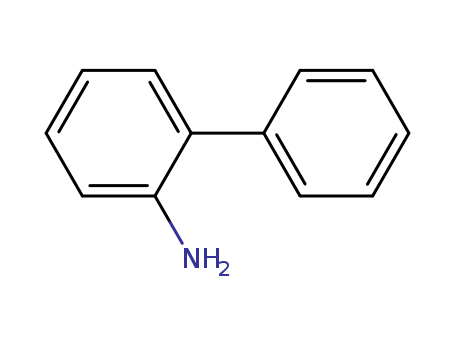
2-phenylaniline

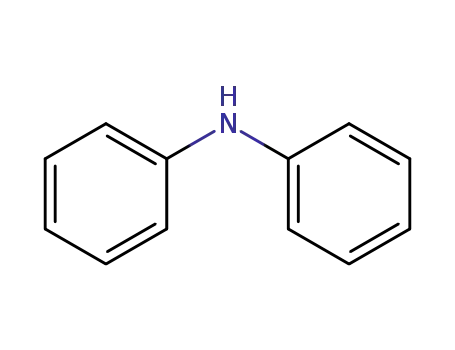
diphenylamine

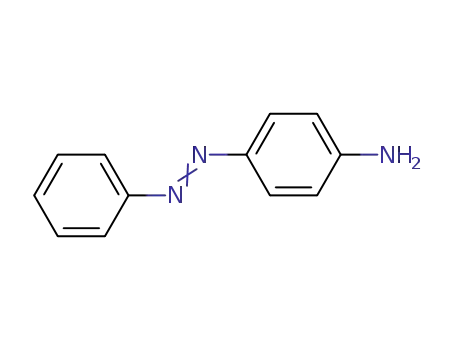
aniline yellow

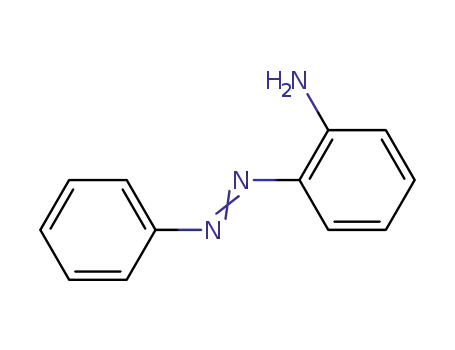
2-aminoazobenzene
| Conditions | Yield |
|---|---|
|
at 21.9 ℃;
for 2.37h;
Kinetics;
Mechanism;
Quantum yield;
Irradiation;
var. of solvent;
|
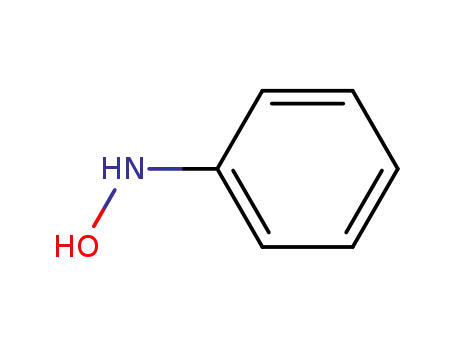
N-Phenylhydroxylamine

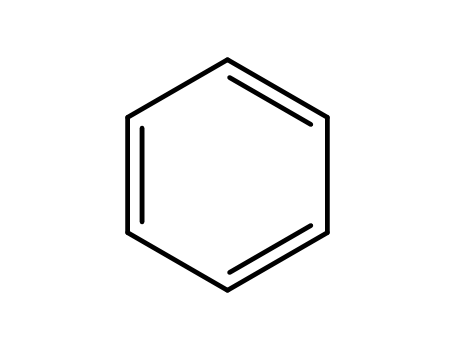
benzene


4-phenylalanine


2-phenylaniline


diphenylamine

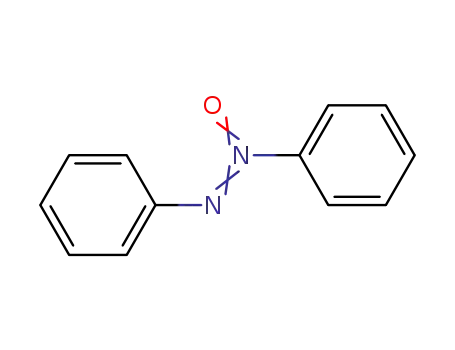
azoxybenzene
| Conditions | Yield |
|---|---|
|
N-Phenylhydroxylamine; benzene;
With
PPA; trifluoroacetic acid;
at 50 ℃;
for 3h;
With
sodium carbonate;
In
water;
|
53 % Chromat. 7.7 % Chromat. 11 % Chromat. |
|
N-Phenylhydroxylamine; benzene;
With
PPA; trifluoroacetic acid;
at 50 ℃;
for 3h;
With
sodium carbonate;
In
water;
|
12 % Chromat. 39 % Chromat. 14 % Chromat. |
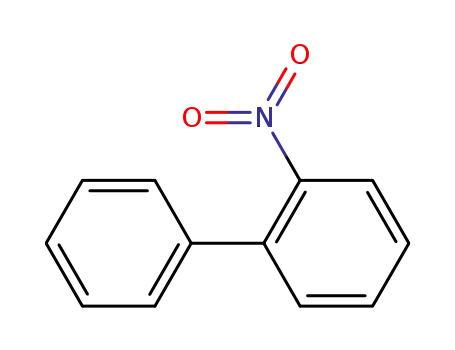
2-nitrobiphenyl
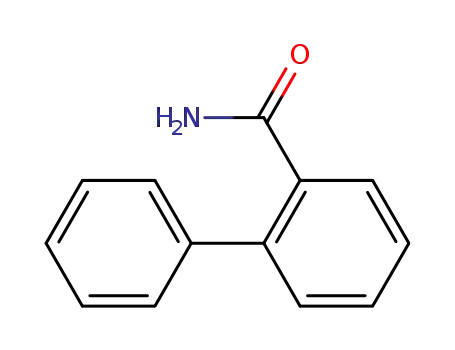
2-phenylbenzamide
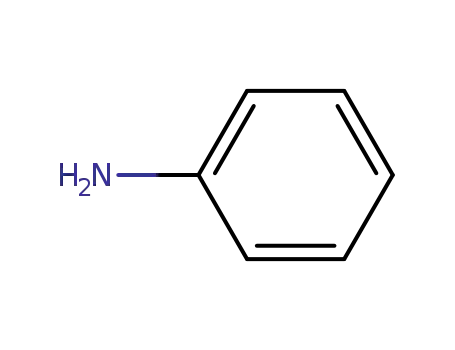
aniline
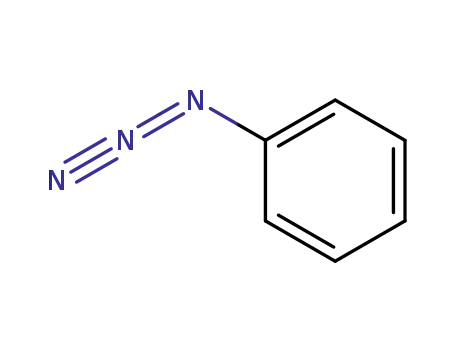
Phenyl azide
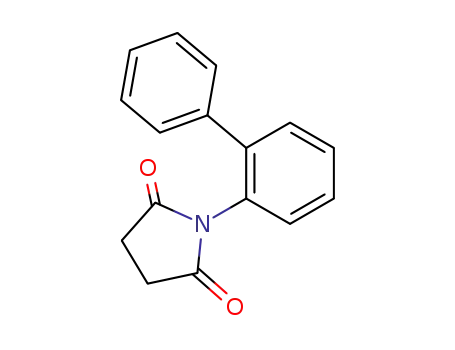
N-biphenyl-succinimide
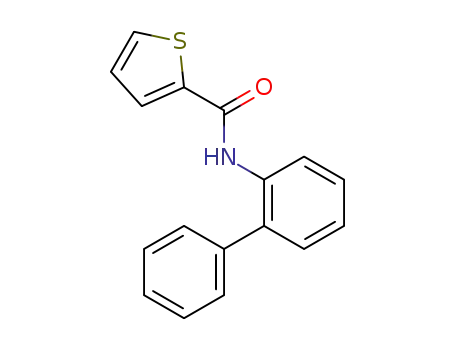
thiophene-2-carboxylic acid biphenyl-2-ylamide
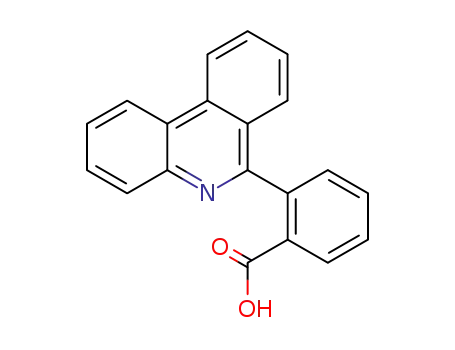
2-phenanthridin-6-yl-benzoic acid
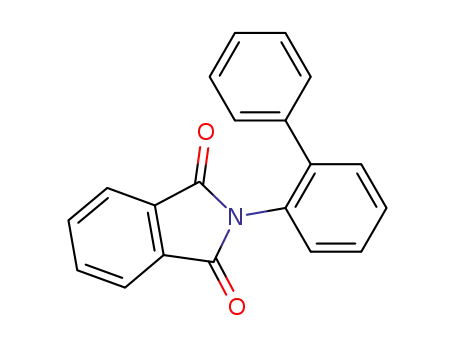
2-(biphenyl-2-yl)-1H-isoindole-1,3(2H)-dione
CAS:333432-28-3
Molecular Formula:C15H15BO2
Molecular Weight:238.09
CAS:1219956-23-6
Molecular Formula:C27H26BN3O2
Molecular Weight:435.3