Your Location:Home >Products >Organic phosphines >CyclohexyI phosphines >829-84-5

Product Details
InChI:InChI=1/C12H23P/c1-3-7-11(8-4-1)13-12-9-5-2-6-10-12/h11-13H,1-10H2
A novel and convenient approach to the synthesis of various tertiary phosphines via a copper-catalyzed cross-coupling of (hetero)aromatic bromide with secondary phosphines has been developed. The reaction employs cheap copper as the catalyst, 2,6-bis(N-methylaminomethyl)pyridine (L4) as a perfect ligand and KOtBu as a base; all reactions are carried out under argon atmosphere. A variety of sterically hindered and/or functionalized substrates were found to react under these reaction conditions to provide products in good to excellent yields. Moreover, ten new tertiary phosphines were first reported in this process.
A rapid method for the reduction of secondary phosphine oxides under mild conditions has been developed, allowing simple isolation of the corresponding free phosphines. The methodology involves the use of pinacol borane (HBpin) to effect the reduction while circumventing the formation of a phosphine borane adduct, as is usually the case with various other commonly used borane reducing agents such as borane tetrahydrofuran complex (BH3?THF) and borane dimethyl sulfide complex (BH3?SMe2). In addition, this methodology requires only a small excess of reducing agent and therefore compares favourably not just with other borane reductants that do not require a metal co-catalyst, but also with silane and aluminium based reagents. (Figure presented.).
The invention relates to a preparation method of a photo-initiator 2,4,6-trimethylbenzoyldicyclohexyloxyphosphine.Under the protection of argon, dicyclohexyl phosphine oxide, metallic sodium and tertiary butanol are reacted in methylbenzene solution to obtain intermediate I; the intermediate I is reacted with 2,4,6-trimethylbenzoyl chloride to obtain intermediate II; the intermediate II is reacted with hydrogen peroxide at 30-35 DEG C to obtain 2,4,6-trimethylbenzoyldicyclohexyloxyphosphine, and liquid phase purity is up to 99.21%.The preparation method is simple to perform and advanced, a solvent is recyclable, and the method is easy for industrial production.
Secondary phosphines, glyoxylic acid hydrate and amines react to form organoammonium phosphonium bis(glycolates) 1a-d. In CD3OD solution, diphenylphosphonium bis(glycolates) undergo reversible solvolysis to phosphinoglycolates 2a,b and acetalic glyoxylic species. The P-dialkyl species 1c avoids this and maintains the phosphonium bis(glycolate) structure in CD 3OD (cHex2P) or undergoes further solvolysis with partial formation of R2PH (R = tBu). Condensation to phosphinoglycines, e.g. 3b, observed for primary amines, does not take place with N-secondary amines at room temperature. Heating leads to condensation but is followed by decarboxylation as shown for the conversion of 2a to 4a. Because of the kinetic lability, the phosphonium compounds 1a-d are sensitive to oxidation by air, H2O2, or sulfur. The resulting phosphinoyl and thiophosphinoyl glycolates and glycolic acids 5-8 are kinetically stable. Precatalyst solutions formed from 1a, c, d and Ni(COD)2 in THF developed moderate to good activity in the oligo- or polymerization of ethylene to linear products containing methyl and vinyl end groups. Activity and molecular weights increased with the +I-effect of the P-substitutents. The solution structures of the novel compounds were elucidated by multinuclear NMR spectroscopy. For 7a a crystal structure analysis is also presented.
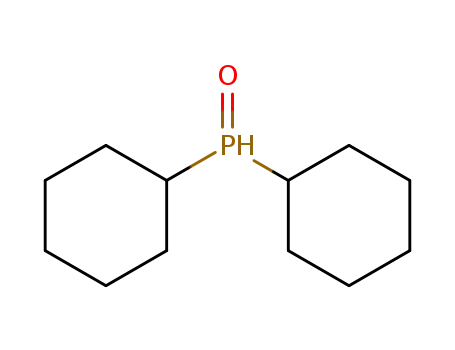
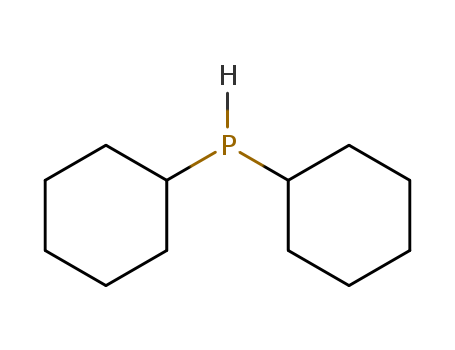
dicyclohexylphosphine oxide

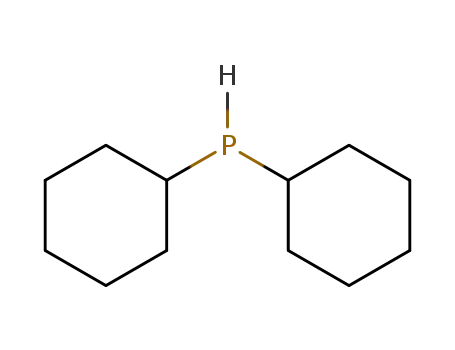
dicyclohexylphosphane
| Conditions | Yield |
|---|---|
|
With
diisobutylaluminium hydride;
In
tetrahydrofuran;
at 50 ℃;
for 4h;
|
88% |
|
dicyclohexylphosphine oxide;
With
diisobutylaluminium hydride;
In
tetrahydrofuran; toluene;
at 20 ℃;
Inert atmosphere;
With
sodium hydroxide;
In
tetrahydrofuran; tert-butyl methyl ether; water; toluene;
at 5 ℃;
|
85% |
|
With
pinacolboronic acid;
In
acetonitrile;
at 20 ℃;
for 20h;
Inert atmosphere;
Glovebox;
Sealed tube;
Schlenk technique;
|
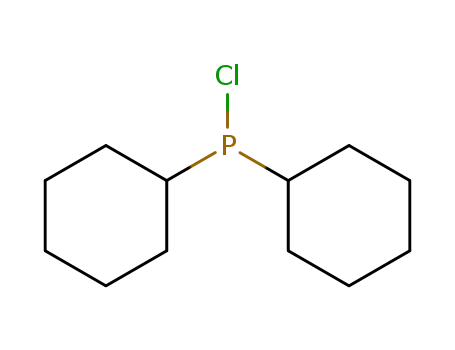
chlorodicyclohexylphosphane


dicyclohexylphosphane
| Conditions | Yield |
|---|---|
|
With
lithium aluminium tetrahydride;
|
|
|
With
diisobutylaluminium hydride;
In
toluene;
at -78 - 23 ℃;
Inert atmosphere;
|
|
|
chlorodicyclohexylphosphane;
In
diethyl ether;
|
|
|
chlorodicyclohexylphosphane;
With
sodium;
In
toluene;
at 100 - 105 ℃;
Inert atmosphere;
With
tert-butyl alcohol;
In
toluene;
at 100 - 105 ℃;
Inert atmosphere;
|
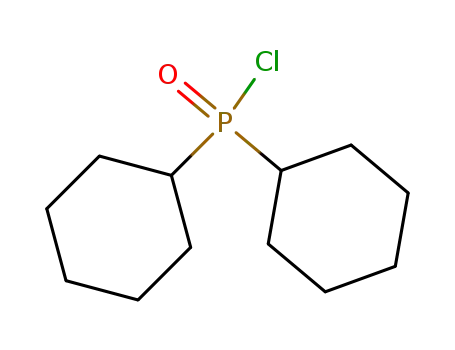
dicyclohexylphosphinoyl chloride
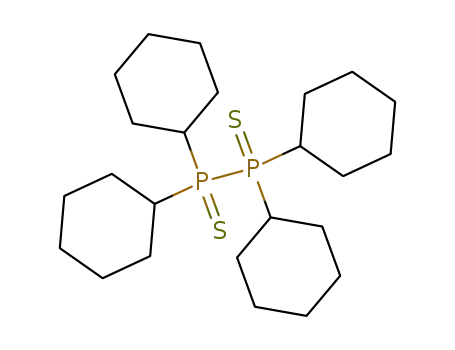
tetracyclohexyl-diphosphane-P,P'-disulfide
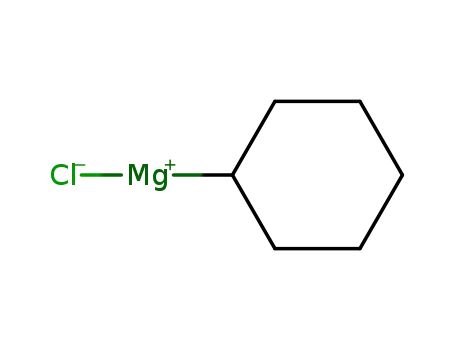
cyclohexylmagnesiumchloride
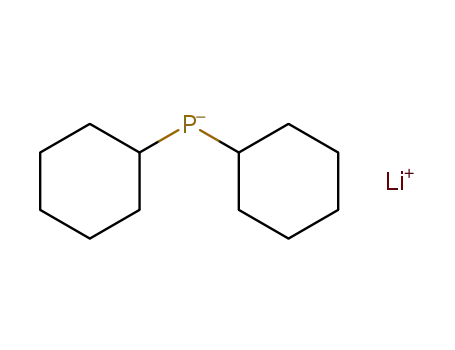
lithium dicyclohexylphosphide
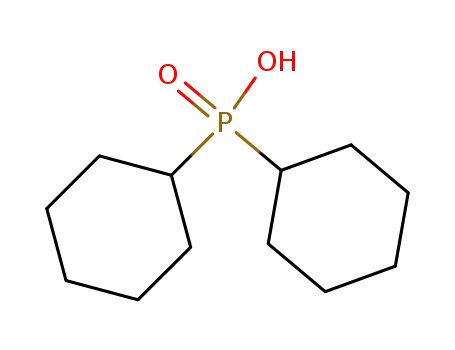
dicyclohexylphosphinic acid
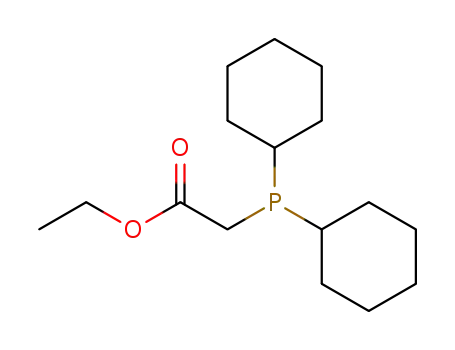
Dicyclohexyl-ethoxycarbonylmethyl-phosphin
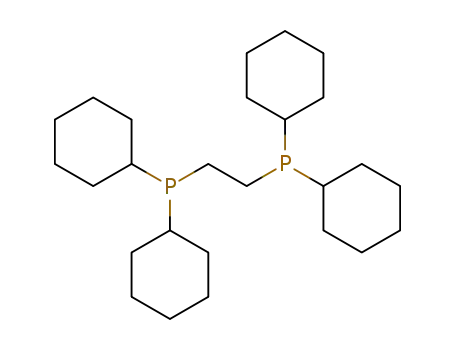
1,2-bis-(dicyclohexylphosphino)ethane
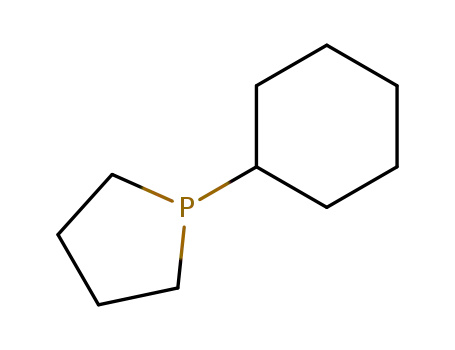
1-cyclohexyl-phospholane
CAS:84680-95-5
CAS:146960-90-9
CAS:108756-88-3