Your Location:Home >Products >Organic phosphines >CyclohexyI phosphines >108756-88-3
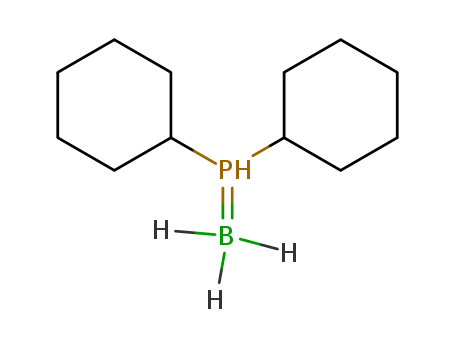

Product Details
Chemical Properties
white powder
We introduce a new reagent class, 2-azatrienes, as a platform for catalytic enantioselective synthesis of allylic amines. Herein, we demonstrate their promise by a diastereodivergent synthesis of syn- and anti-1,2-diamines through their Cu-bis(phosphine)-catalyzed reductive couplings with imines. With Ph-BPE as the supporting ligand, anti-diamines are obtained (up to 91% yield, >20:1 dr, and >99:1 er), and with the rarely utilized t-Bu-BDPP, syn-diamines are generated (up to 76% yield, 1:>20 dr, and 97:3 er).
(Dicyclohexylphosphino)borane is a precursor in the synthesis of water-soluble phosphines. The boranato group can be removed quantitatively under mild conditions.
An enantioselective consecutive cyclization/coupling process, catalyzed by palladium is reported. Stereoinduction arises from an enantioselective carbopalladation, generating an intermediate which promotes a nucleopalladation step. The dual cyclization se
The purpose of the present invention is to provide an N,N-bis(2-dialkylphosphinoethyl)amine-borane complex which is a ruthenium complex that exhibits excellent catalytic activity in a hydrogenation reaction, etc., and a production method therefor, and a method for efficiently producing a ruthenium complex containing N,N-bis(2-dialkylphosphinoethyl)amine as a ligand. The present invention is capable of efficiently producing an amine-borane complex (3) by reacting an oxazolidinone compound (1) with a dialkylphosphine-borane compound (2) in the presence of a base. The present invention is also capable of efficiently producing a ruthenium complex (5) by reacting the amine-borane complex (3) with a ruthenium compound (4) in the presence of an amine. (In the formula, a solid line, a dashed line, B, C, H, L1-L3, LG, n, N, O, P, Ru, X, and R1-R10 are as defined in the description.)
The present invention relates to: a compound as a ligand in a variety of catalytic organic synthetic reactions; a method for producing the compound; a synthetic intermediate of the compound; and a transition metal complex which has the compound as a ligand. The compound includes a compound represented by the following general formula (1A):
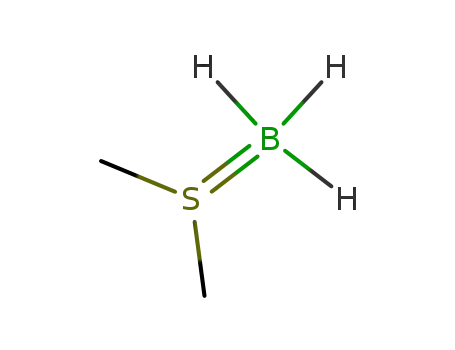
dimethylsulfide borane complex

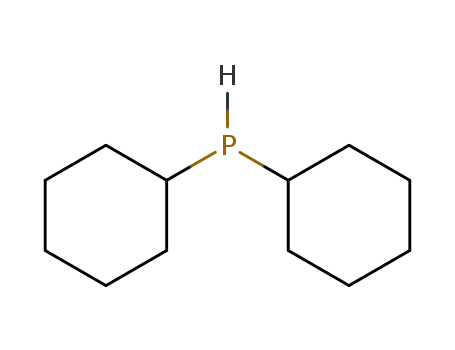
dicyclohexylphosphane

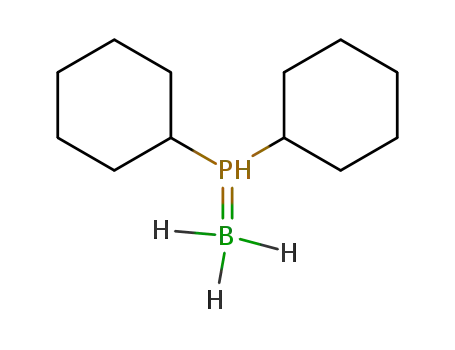
(dicyclohexylphosphino)borane
| Conditions | Yield |
|---|---|
|
In
diethyl ether;
at 5 - 10 ℃;
for 0.166667h;
Cooling with ice;
|
100% |
|
In
diethyl ether;
at 5 - 20 ℃;
for 0.166667h;
Inert atmosphere;
|
19.3 g |
|
In
diethyl ether;
at 0 - 20 ℃;
Inert atmosphere;
|
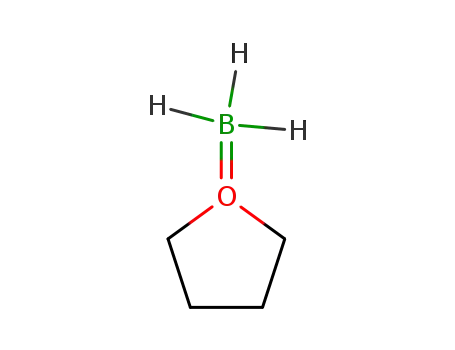
borane-THF


dicyclohexylphosphane


(dicyclohexylphosphino)borane
| Conditions | Yield |
|---|---|
|
In
tetrahydrofuran;
(Ar), borane tetrahydrofuran soln. slowly addn. to dicyclohexylphosphinesoln. (273 K), stirring (273 K, 2 h), warming (room temp.), crystn. on evapn.; recrystn. (pentane);
|
90% |
|
In
tetrahydrofuran;
at -78 ℃;
for 16h;
Schlenk technique;
Inert atmosphere;
|
81% |
|
In
tetrahydrofuran;
at 0 - 20 ℃;
for 1h;
|
|
|
In
tetrahydrofuran;
identified by NMR;
|
|
|
In
tetrahydrofuran;
at 5 - 10 ℃;
for 0.5h;
|

dicyclohexylphosphane
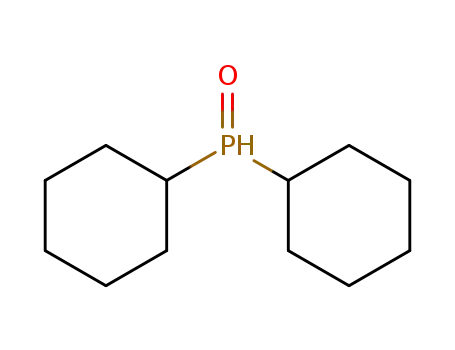
dicyclohexylphosphine oxide

borane-THF
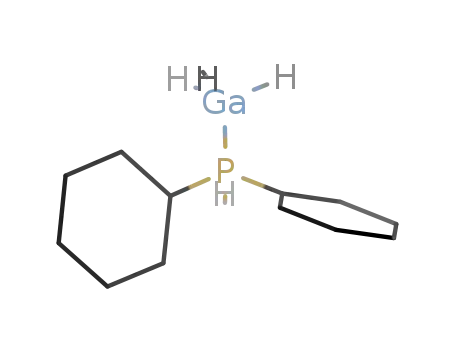
Cy2PH*GaH3
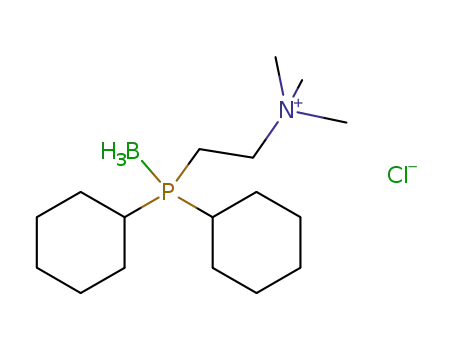
(CH3)3NC2H4P(C6H11)2(BH3)(1+)*Cl(1-)=[(CH3)3NC2H4P(C6H11)2(BH3)]Cl
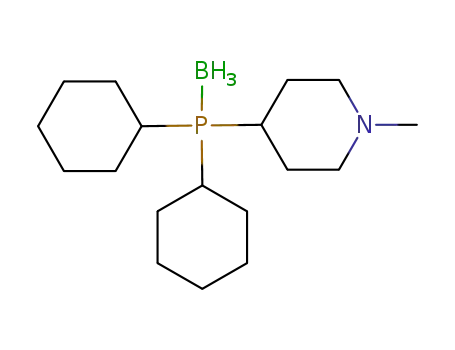
(C6H11)2P(BH3)C5H9NCH3
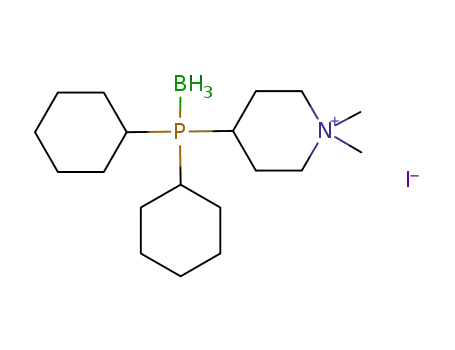
(C6H11)2P(BH3)C5H9N(CH3)2(1+)*I(1-)=[(C6H11)2P(BH3)C5H9N(CH3)2]I
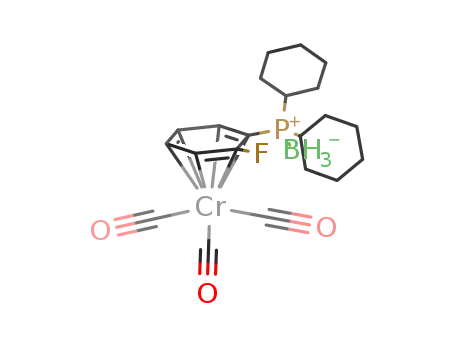
2-(boranatodicyclohexylphosphino)fluorobenzenetricarbonylchromium
CAS:300-57-2
CAS:829-84-5
CAS:19999-87-2