Your Location:Home >Products >OLED intermediates >Thiophenes >145543-82-4
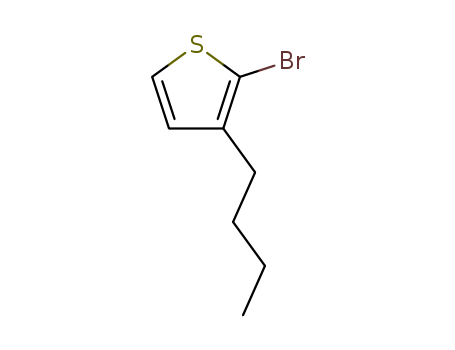

Product Details
InChI:InChI=1/C8H11BrS/c1-2-3-4-7-5-6-10-8(7)9/h5-6H,2-4H2,1H3
Two-dimensional covalent organic frameworks (2D-COFs) are crystalline, porous materials comprising aligned columns of π-stacked building blocks. With a view toward the application of these materials in organic electronics and optoelectronics, the construction of oligothiophene-based COFs would be highly desirable. The realization of such materials, however, has remained a challenge, in particular with respect to laterally conjugated imine-linked COFs. We have developed a new building block design employing an asymmetric modification on an otherwise symmetric backbone that allows us to construct a series of highly crystalline quaterthiophene-derived COFs with tunable electronic properties. Studying the optical response of these materials, we have observed for the first time the formation of a charge transfer state between the COF subunits across the imine bond. We believe that our new building block design provides a general strategy for the construction of well-ordered COFs from various extended building blocks, thus greatly expanding the range of applicable molecules.
The isomers of 3,7-dimethyl-2,6-octadienal, more commonly known together as citral, are two of the most notable natural compounds in the flavor and fragrance industry. However, both isomers are inherently unstable, limiting their potential use in various applications. To identify molecules in nature that can impart the fresh lemon character of citral while demonstrating stability under acidic and thermal conditions has been a major challenge and goal for the flavor and fragrance industry. In the study of fried chicken, several alkyl thiophenecarbaldehydes were identified by gas chromatography-mass spectrometry and gas chromatography-olfactometry that provided a similar citral-like aroma. The potential mechanism of formation in fried chicken is discussed. Furthermore, in order to explore the organoleptic properties of this structural backbone, a total of 35 thiophenecarbaldehyde derivatives were synthesized or purchased for evaluation by odor and taste. Certain organoleptic trends were observed as the length of the alkyl or alkenyl chain increased or when the chain was moved to different positions on the thiophene backbone. The 3-substituted alkyl thiophenecarbaldehydes, specifically 3-butyl-2-thiophenecarbaldehyde and 3-(3-methylbut-2-en-1-yl)-2-thiophenecarbaldehyde, exhibited strong citrus and citral-like notes. Several alkyl thiophenecarbaldehydes were tested in high acid stability trials (4 °C vs 38 °C) and outperformed citral both in terms of maintaining freshness over time and minimizing off-notes. Additional measurements were completed to calculate the odor thresholds for a select group of thiophenecarbaldehydes, which were found to be between 4.7-215.0 ng/L in air.
Bromination in concentrated solutions of substituted thiophenes in acetic acid with NBS at room temperature was studied. Under the conditions, bromination readily took place with high regioselectivity at the 2-ring positions, > 99%. The developed method was demonstrated to be suitable for multimolar preparations.
A systematically regiocontrolled synthesis of poly(3-alkylthiophenes) (P3AT) mediated by Rieke zinc is reported. Rieke zinc undergoes oxidative addition to 2,5-dibromo-3-alkylthiophene or 2-bromo-5-iodo-3-alkylthiophene regioselectively to afford 2-bromo-5-(bromozincio)-3-alkylthiophene (2) or 2-bromo-5-(iodozincio)-3-alkylthiophene (10). The intermediate 2 or 10 can be polymerized catalytically to a series of regiospecific poly(3-alkylthiophenes) using different catalysts. The regioregularity of the polymer chain is solely controlled by the structure of the catalyst. An almost completely regioregular head-to-tail (HT) P3AT (4) is obtained by using Ni(DPPE)Cl2 ([1,2-bis-(diphenylphosphino)ethane]nickel(II) chloride). Use of Pd(DPPE)Cl2 leads to a reduction in the regioregularity (70: 30 HT/HH), while using Ni(PPh3)4 also leads to a much reduced regioregular P3AT (63:35 HT/HH). A totally regiorandom (50:50 HT/HH) P3AT (5) is afforded by using Pd(PPh3)4. The poly(3-butylthiophene) 4a is a 97% HT regioregular polymer. Other poly(3-alkylthiophenes) (alkyl = hexyl (4b), octyl (4c), decyl (4d), dodecyl (4e), and tetradecyl (4f)) are regioregular P3ATs with the HT linkage larger than 98.5% based on NMR analysis. Electronic absorption, X-ray diffraction, and crossed polarizing micrograph studies show that the cast films of the regioregular P3ATs (4) are self-organized, crystalline, flexible, and bronze-colored films with a metallic luster, while that of the regiorandom P3ATs (5) are amorphous and orange-colored films. The regioregular P3ATs exhibit a small bandgap (1.7 eV) which is 0.4 eV lower than that of regiorandom P3ATs (2.1 eV). Regioregular HT P3ATs have considerably improved electroconductivity and other physical properties over regiorandom P3ATs.
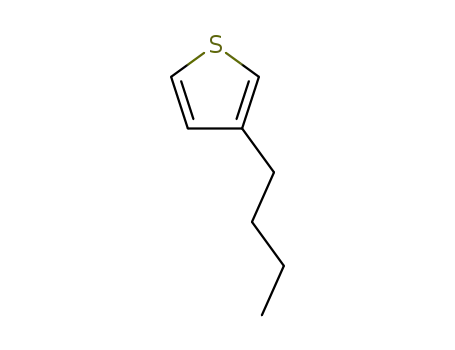
3-butylthiophene

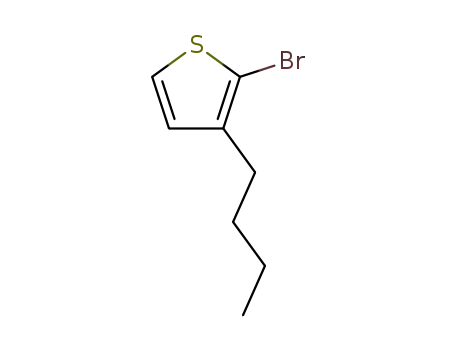
2-bromo-3-(n-butyl)thiophene

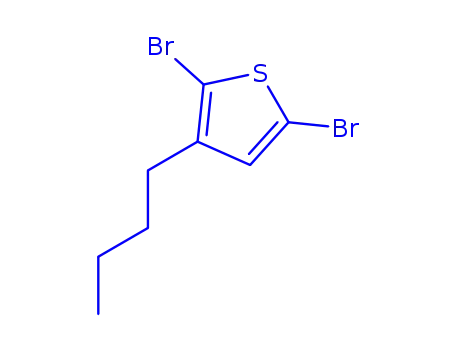
2,5-dibromo-3-butylthiophene
| Conditions | Yield |
|---|---|
|
With
N-Bromosuccinimide;
In
acetic acid;
for 0.15h;
Yields of byproduct given;
|
96.9% |

3-butylthiophene


2-bromo-3-(n-butyl)thiophene
| Conditions | Yield |
|---|---|
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform;
at 0 - 20 ℃;
for 48h;
Darkness;
Inert atmosphere;
Schlenk technique;
|
89% |
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
at 20 - 50 ℃;
for 0.5h;
|
79% |
|
With
bromine;
In
acetic acid;
at 0 ℃;
for 0.5h;
|
48% |
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform;
at 20 ℃;
for 0.5h;
|

3-butylthiophene
3-butyl-2-(3,4-dimethoxyphenyl)thiophene

3,3'-dibutyl-2,2'-bithiophene

C44H42O2S2
CAS:1189047-28-6
CAS:28320-31-2
Molecular Formula:C<sub>15</sub>H<sub>13</sub>Br
Molecular Weight:273.17
CAS:116971-10-9
CAS:34722-01-5