Your Location:Home >Products >Organic phosphines >Phenyl phosphines >6476-37-5
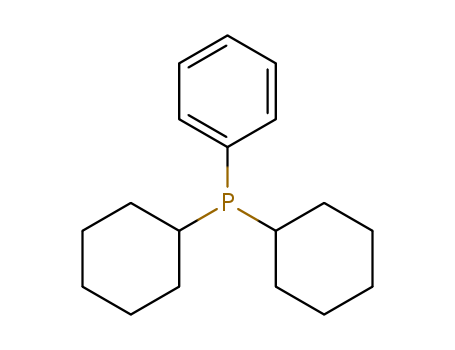

Product Details
Chemical Properties
White to off-white crystalline powder
Uses
Dicyclohexylphenylphosphine ?is an organic phosphine compound, which can be used as a raw material for organic synthesis.
InChI:InChI=1/C18H27P/c1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18/h1,4-5,10-11,17-18H,2-3,6-9,12-15H2
Poly(4-diphenylphosphinostyrene) (1), poly(4-dicyclohexylphosphinostyrene) (2), and poly (3) were obtained by polymerization of the corresponding monomers.The polymers were treated with (RhClL2)2 and with (RhClL')2, where L and L' are ethene and 1,5-cyclooctadiene, respectively.These complexes were added stepwise and during this process the 31P NMR spectra were observed. 31P chemical shifts and 103Rh-31P coupling constants were compared with those of monomeric analogues which were treated in the same way.
The kinetics of quinuclidine displacement of BH3 from a wide range of Lewis base borane adducts have been measured. Parameterization of these rates has enabled the development of a nucleofugality scale (NFB), shown to quantify and predict the leaving group ability of a range of other Lewis bases. Additivity observed across a number of series R′3-nRnX (X = P, N; R′ = aryl, alkyl) has allowed the formulation of related substituent parameters (nfPB, nfAB), providing a means of calculating NFB values for a range of Lewis bases that extends far beyond those experimentally derived. The utility of the nucleofugality parameter is explored by the correlation of the substituent parameter nfPB with the hydrolyses rates of a series of alkyl and aryl MIDA boronates under neutral conditions. This has allowed the identification of MIDA boronates with heteroatoms proximal to the reacting center, showing unusual kinetic lability or stability to hydrolysis.
Palladium-catalyzed C-P bond formation reaction of ArBr/ArOTf using acylphosphines as differential phosphination reagents is reported. The acylphosphines show practicable reactivity with ArBr and ArOTf as the phosphination reagents, though they are inert to the air and moisture. The reaction affords trivalent phosphines directly in good yields with a broad substrate scope and functional group tolerance. This reaction discloses the acylphosphines' capability as new phosphorus sources for the direct synthesis of trivalent phosphines.
Asymmetrically substituted tertiary phosphines and quaternary phosphonium salts are used extensively in applications throughout industry and academia. Despite their significance, classical methods to synthesize such compounds often demand either harsh reaction conditions, prefunctionalization of starting materials, highly sensitive organometallic reagents, or expensive transition-metal catalysts. Mild, practical methods thus remain elusive, despite being of great current interest. Herein, we describe a visible-light-driven method to form these products from secondary and primary phosphines. Using an inexpensive organic photocatalyst and blue-light irradiation, arylphosphines can be both alkylated and arylated using commercially available organohalides. In addition, the same organocatalyst can be used to transform white phosphorus (P4) directly into symmetrical aryl phosphines and phosphonium salts in a single reaction step, which has previously only been possible using precious metal catalysis.
Aryl(dicyclohexyl)phosphines were prepared by a catalytic C-P bond-forming cross-coupling reaction of haloarenes with dicyclohexylphosphine under heterogeneous conditions in water containing an immobilized palladium complex coordinated to an amphiphilic polystyrene-poly(ethylene glycol) resin supported di(tert -butyl)phosphine ligand.
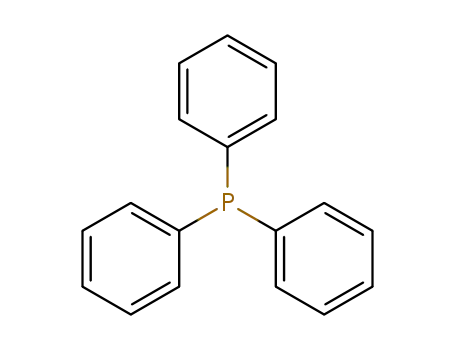
triphenylphosphine

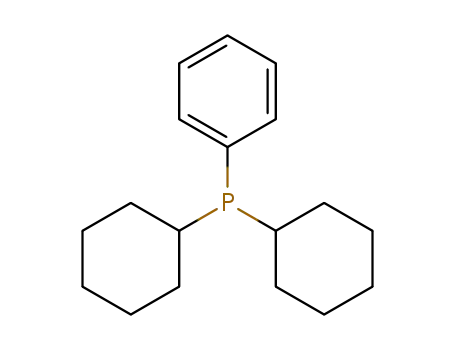
dicyclohexylphenylphosphine

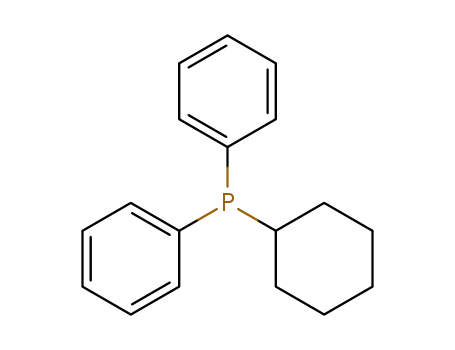
cyclohexyldiphenylphosphine

tricyclohexylphosphine
| Conditions | Yield |
|---|---|
|
With
n-butyllithium; hydrogen;
bis(2,6-diisopropylphenoxy) trichloroniobium(V);
In
hexane; benzene;
at 60 ℃;
under 62057.8 Torr;
Kinetics;
var. of catalyst;
|
|
|
With
n-butyllithium; hydrogen;
|
Dichlorophenylphosphine

cyclohexylmagnesium bromide


dicyclohexylphenylphosphine
| Conditions | Yield |
|---|---|
|
In
tetrahydrofuran;
|
cyclohexyllithium

Dichlorophenylphosphine
chlorobenzene
lithium dicyclohexylphosphide
di(cyclohexyl)phenylphosphine oxide
dicyclohexylphenylphosphine selenide
dicyclohexyl(phenyl)phosphine sulfide

<(E)-1-iodo-1-penten-5-yl>dicyclohexylphenylphosphonium iodide
CAS:829-84-5
CAS:6372-42-5
CAS:2622-14-2