Your Location:Home >Products >Organic phosphines >Phenyl phosphines >6372-42-5
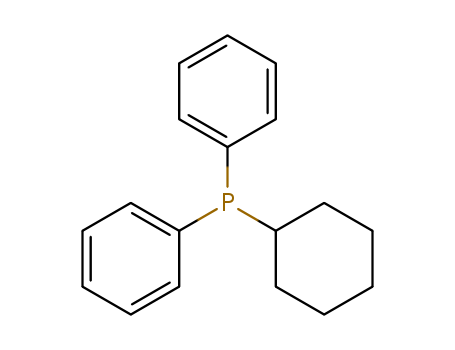

Product Details
Chemical Properties
white to light yellow crystal powde
Uses
suzuki reaction
Uses
Cyclohexyldiphenylphosphine is used as ligand in coupling reaction. It is also used as a medical intermediate.
InChI:InChI=1/C18H21P/c1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18/h1-2,4-7,10-13,18H,3,8-9,14-15H2
The invention aims to provide an aryl phosphine oxide compound as a raw material, wherein P=O keys are activated by an acid anhydride and alkali is continued. The preparation of the phosphine (III) compound is carried out under the action of a crown ether and a reducing agent. The method has the advantages of cheap and easily available raw materials, simple operation, high atomic economy and the like. Compared with a traditional reduction mode, the method is ingenious in design, waste emission is reduced, separation of intermediate products is omitted, and related reagents such as silicon hydrogen, aluminum, boron and the like with higher price can be avoided. And the reaction suitability is extensive.
The kinetics of quinuclidine displacement of BH3 from a wide range of Lewis base borane adducts have been measured. Parameterization of these rates has enabled the development of a nucleofugality scale (NFB), shown to quantify and predict the leaving group ability of a range of other Lewis bases. Additivity observed across a number of series R′3-nRnX (X = P, N; R′ = aryl, alkyl) has allowed the formulation of related substituent parameters (nfPB, nfAB), providing a means of calculating NFB values for a range of Lewis bases that extends far beyond those experimentally derived. The utility of the nucleofugality parameter is explored by the correlation of the substituent parameter nfPB with the hydrolyses rates of a series of alkyl and aryl MIDA boronates under neutral conditions. This has allowed the identification of MIDA boronates with heteroatoms proximal to the reacting center, showing unusual kinetic lability or stability to hydrolysis.
The direct and scalable electroreduction of triphenylphosphine oxide (TPPO)-the stoichiometric byproduct of some of the most common synthetic organic reactions-to triphenylphosphine (TPP) remains an unmet challenge that would dramatically reduce the cost and waste associated with performing desirable reactions that are mediated by TPP on a large scale. This report details an electrochemical methodology for the single-step reduction of TPPO to TPP using an aluminum anode in combination with a supporting electrolyte that continuously regenerates a Lewis acid from the products of anodic oxidation. The resulting Lewis acid activates TPPO for reduction at mild potentials and promotes P-O over P-C bond cleavage to selectively form TPP over other byproducts. Finally, this robust methodology is applied to (i) the reduction of synthetically useful classes of phosphine oxides, (ii) the one-pot recycling of TPPO generated from a Wittig reaction, and (iii) the gram-scale reduction of TPPO at high concentration (1 M) with continuous product extraction and in flow at high current density.
Asymmetrically substituted tertiary phosphines and quaternary phosphonium salts are used extensively in applications throughout industry and academia. Despite their significance, classical methods to synthesize such compounds often demand either harsh reaction conditions, prefunctionalization of starting materials, highly sensitive organometallic reagents, or expensive transition-metal catalysts. Mild, practical methods thus remain elusive, despite being of great current interest. Herein, we describe a visible-light-driven method to form these products from secondary and primary phosphines. Using an inexpensive organic photocatalyst and blue-light irradiation, arylphosphines can be both alkylated and arylated using commercially available organohalides. In addition, the same organocatalyst can be used to transform white phosphorus (P4) directly into symmetrical aryl phosphines and phosphonium salts in a single reaction step, which has previously only been possible using precious metal catalysis.
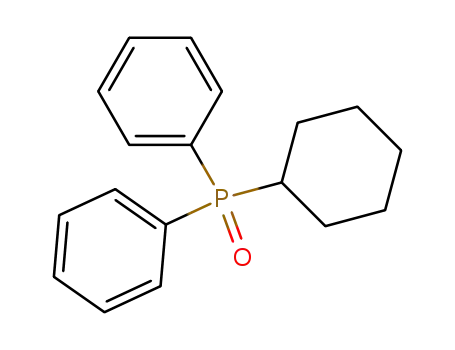
(cyclohexyl)diphenylphosphane oxide

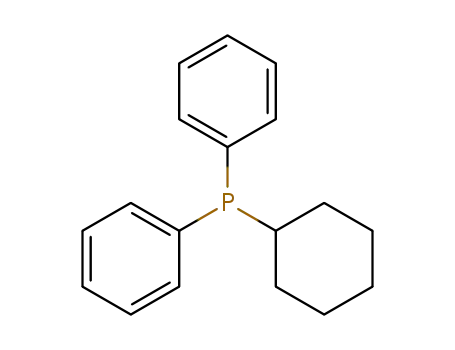
cyclohexyldiphenylphosphine
| Conditions | Yield |
|---|---|
|
With
copper(II) trifluoromethanesulfonate; 1,1,3,3-Tetramethyldisiloxane;
In
toluene;
at 100 ℃;
Inert atmosphere;
|
86% |
|
With
oxalyl dichloride; hydrogen;
In
chloroform-d1;
at 130 ℃;
for 18h;
under 60006 Torr;
Reagent/catalyst;
|
85% |
|
(cyclohexyl)diphenylphosphane oxide;
With
trifluoroacetic anhydride;
In
1,4-dioxane;
at 20 ℃;
for 0.5h;
Inert atmosphere;
With
15-crown-5; sodium hydride; sodium hydrogencarbonate;
In
1,4-dioxane;
at 150 ℃;
for 24h;
Inert atmosphere;
|
85% |
|
With
copper(II) bis(trifluoromethanesulfonate); 1,1,3,3-tetramethyldisilazane;
In
toluene;
at 80 ℃;
|
82% |
|
With
copper(II) bis(trifluoromethanesulfonate); 1,1,3,3-tetramethyldisilazane;
In
toluene;
at 80 ℃;
|
82% |
|
With
triphenyl phosphite; diphenyl hydrogen phosphite; iodine;
In
tetrahydrofuran;
at 20 ℃;
Inert atmosphere;
|
75% |
|
With
oxalyl dichloride; diethyl 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate; triethylamine;
In
dichloromethane;
at 40 ℃;
for 2h;
Inert atmosphere;
|
73% |
|
With
diethoxymethylane; Bis(p-nitrophenyl) phosphate;
In
toluene;
at 110 ℃;
chemoselective reaction;
Inert atmosphere;
|
70% |
|
With
aluminum (III) chloride; N,N,N,N,-tetramethylethylenediamine; tetrabutyl-ammonium chloride; tert-butylammonium hexafluorophosphate(V);
In
acetonitrile;
at -20 ℃;
Inert atmosphere;
Glovebox;
Electrolysis;
|
45% |
|
(cyclohexyl)diphenylphosphane oxide;
With
[Fe(CO)(1,3-bis(diphenylphosphino)propane)H(NO)]; phenylsilane; N-ethyl-N,N-diisopropylamine;
In
toluene;
at 100 ℃;
for 18h;
Schlenk technique;
With
sodium hydroxide;
In
methanol; water; toluene;
at 20 ℃;
for 1h;
chemoselective reaction;
Schlenk technique;
|
44% |
|
With
indium(III) bromide; 1,1,3,3-Tetramethyldisiloxane;
In
toluene;
at 100 ℃;
for 22h;
Inert atmosphere;
Sealed tube;
|
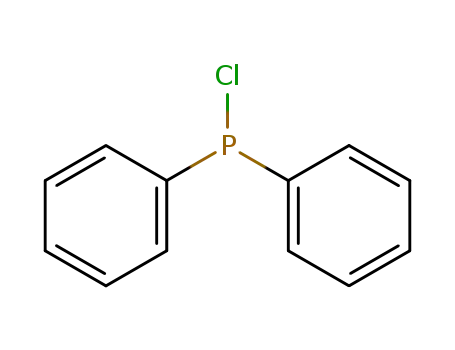
chloro-diphenylphosphine

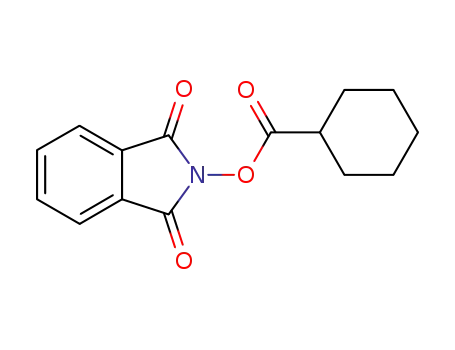
1,3-dioxoisoindolin-2-yl cyclohexanecarboxylate


cyclohexyldiphenylphosphine
| Conditions | Yield |
|---|---|
|
With
N,N,N',N'',N'''-pentamethyldiethylenetriamine; zinc;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 12h;
Reagent/catalyst;
Time;
|
95% |
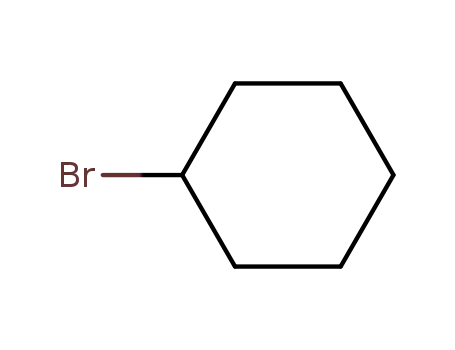
1-bromocyclohexane
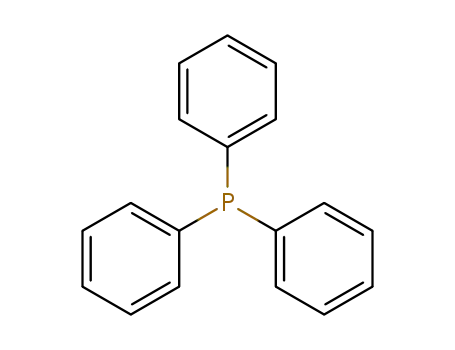
triphenylphosphine
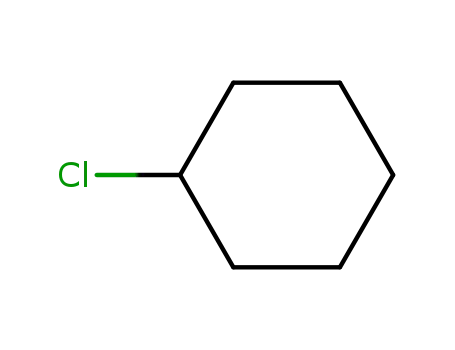
cyclohexyl chloride

dicyclohexylzinc(II)
Dichlor(cyclohexyl)diphenylphosphoran
1,4-di(cyclohexylphenylphosphino)butane

(cyclohexyl)diphenylphosphane oxide
cyclohexyl(diphenyl)phosphine sulfide
CAS:27721-02-4
CAS:577-19-5
CAS:16523-54-9
CAS:6476-37-5