Your Location:Home >Products >OLED intermediates >Carbazoles >1592-95-6

Product Details
|
Description |
3-Bromo-9H-carbazole is a white to light yellow solid used as an intermediate for organic light-emitting diodes (OLEDs) and pharmaceuticals. |
|
Uses |
3-Bromo-9H-carbazole functions as a bipolar host material with high triplet energy, making it suitable for use in blue phosphorescent OLEDs, enhancing device efficiency and stability. Additionally, it serves as an aryl hydrocarbon receptor agonist and is used as a standard for environmental testing and research. Its derivatives, due to bromination at the 3-position, have reduced chances of oxidation, which is advantageous for forming stable materials in advanced electronic applications. |
|
Preparation |
3-Bromocarbazole synthesis: A solution of N-bromosuccinimide (1.1g, 5.98mmol) in dimethylformamide was added dropwise to a solution of carbazole (1, 1g, 5.96mmol) in dimethylformamide (15mL) at 0°C. The reaction mixture was then stirred at room temperature for 24h. The reaction was poured into distilled water to give a cream coloured precipitate. The precipitate was filtered off under vacuum and washed with distilled water (3 × 20mL). The precipitate was dissolved in ethyl acetate, dried with sodium sulfate and filtered. Upon concentration under reduced pressure the crude product was obtained as a brown solid. After crystallisation of the crude product with chloroform, the pure 3-Bromo-9H-carbazole(692 mg, 47%) was obtained as white crystals. Rf (ethyl acetate/hexane, 1:6 v/v): 0.43; melting point: 200–201°C. |
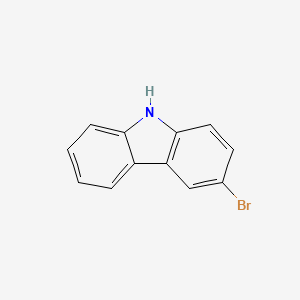
Isomeric SMILES: C1=CC=C2C(=C1)C3=C(N2)C=CC(=C3)Br
InChIKey: LTBWKAYPXIIVPC-UHFFFAOYSA-N
InChI: InChI=1S/C12H8BrN/c13-8-5-6-12-10(7-8)9-3-1-2-4-11(9)14-12/h1-7,14H
In this Letter, we investigated the barr...
However, PHCZs such as 3-chloro-9Hcarbazole (3-CCZ), 3-bromo-9H-carbazole (3-BCZ), … 36dibromo-9H-carbazole and 3-bromo-9H-carbazole are potential AR antagonists, with the …
A ruthenium–nitrosyl derivative of formu...
We have been developing the methodology ...
In this work, four main chain polymeric ...
4-(9′-Hexylcarbazol-3′-yl)benzaldehyde (...
Bromoeudistomin D and 9-methyl-7-bromoeu...
This paper reported a two-photon fluores...
Two novel charged organic thermally acti...
A new biscarbazoloanthracene consisting ...
Two novel carbazole-based compounds 7a a...
Two solution-processable, carbazole-base...
The synthesis of novel diblock polymers ...
Herein, regioselectivepara-C-H halogenat...
6-bromo-1,2,3,4-tetrahydro-9H-carbazole
1-(3-bromo-9H-carbazol-9-yl)ethan-1-one
9-benzyl-9H-carbazole
(3-bromo-9H-carbazol-9-yl)(phenyl)methanone
(3-bromo-9H-carbazol-9-yl)(phenyl)methanone
3-Hydroxycarbazol
9H-carbazole-3-carboxylic acid
3-methoxycarbazole
CAS:15155-41-6
Molecular Formula:C6H2Br2N2S
Molecular Weight:293.97
CAS:16807-13-9