Your Location:Home >Products >Organic phosphines >CyclohexyI phosphines >16523-54-9
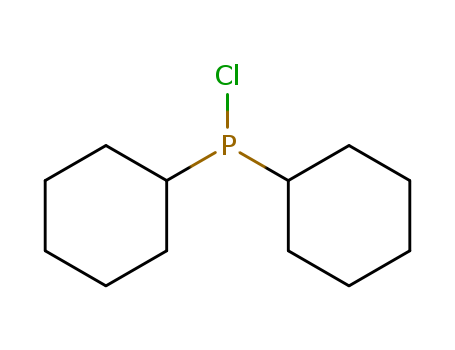

Product Details
Chemical Properties
clear colorless liquid
Uses
Dicyclohexylchlorophosphine is used as pharmaceutical intermediate. It is also used in the synthesis of various phosphines.
Uses
Chlorodicyclohexylphosphine can be used as a reactant in the synthesis of: 1,2-Bis(dicyclohexylphosphinoxy)ethane ligand by reacting with ethylene glycol in the presence of triethylamine via Michaelis?Arbuzov type rearrangements.1,1,2,2-tetracyclohexyldiphosphine monosulfide ligand by treating with LiS.It can be also used as a starting material for the preparation of some other ligands such as dicyclohexylphosphine oxide , phosphino substituted N-aryl pyrroles , di-tert-butyl((dicyclohexylphosphino)methyl)phosphine , dicyclohexylcyclopentylphosphine. These ligands are used in Pd-catalyzed cross-coupling reactions.
Application
Chlorodicyclohexylphosphine can be used as a reactant in the synthesis of:1,2-Bis(dicyclohexylphosphinoxy)ethane ligand by reacting with ethylene glycol in the presence of triethylamine via Michaelis?Arbuzov type rearrangements.1,1,2,2-tetracyclohexyldiphosphine monosulfide ligand by treating with LiS.It can be also used as a starting material for the preparation of some other ligands such as dicyclohexylphosphine oxide , phosphino substituted N-aryl pyrroles , di-tert-butyl((dicyclohexylphosphino)methyl)phosphine , dicyclohexylcyclopentylphosphine. These ligands are used in Pd-catalyzed cross-coupling reactions.
Precautions
Store in cool, dry conditions in well sealed containers. Protect from humidity and water. Store under dry inert gas. It is sensitive to air and moisture. Incompatible with strong oxidizing agents.
InChI:InChI=1/C12H22ClP/c13-14(11-7-3-1-4-8-11)12-9-5-2-6-10-12/h11-12H,1-10H2
Biphenyl-based phosphine ligands can be prepared on a significantly larger scale than previously possible as a result of the following discoveries and improvements to the original experimental procedure: the finding that CuCl catalyzes the coupling of hindered dialkylchlorophosphines with Grignard reagents; the development of conditions that permit ClPCy2 to be prepared and utilized in situ; the development of a more reliable large-scale preparation of 2-dimethylaminophenylmagnesium halide.
The present invention relates to the compound of dialkyl(2-alkoxy-6-aminophenyl)phosphine and the preparation method thereof and the application in the palladium catalyzed coupling reactions of aryl chloride and ketone. The dialkyl(2-alkoxy-6-aminophenyl)phosphine of the present invention could coordinate with the palladium catalyst to highly selectively activate the inert carbon-chlorine bond, and to catalyze direct arylation reaction in the α-position of ketones to produce corresponding coupling compounds. The preparation method of the present invention is a simple one-step method which produces the air-stable dialkyl(2-alkoxy-6-aminophenyl)phosphine. Compared with the synthetic routes of ligands to be used in the activation of carbon-chlorine bonds in the prior arts, the preparation method of the present invention has the advantages of short route and easy operation.
A new, readily available, and air-stable monophosphine ligand, i.e., Zheda-Phos, has been developed for the general and highly effective palladium-catalyzed monoarylation of acetone with aryl chlorides. The reaction rate is of first-order dependence with the aryl chloride. Copyright
The current invention relates to the structure, synthesis of dialkyl(2,4,6- or 2,6-alkoxyphenyl)phosphine or its tetrafluoroborate, as well as its applications in the palladium catalyzed carbon-chlorine bond activation for Suzuki coupling reactions and carbon-nitrogen bond formation reactions. The dialkyl(2,4,6- or 2,6-alkoxyphenyl)phosphine or its tetrafluoroborate could coordinate with the palladium catalyst to activate the inert carbon-chlorine bond highly selectively and catalyze Suzuki coupling reaction with arylboronic acid or carbon-nitrogen bond formation reaction with organic amines. The current invention uses only one step to synthesize dialkyl(2,4,6- or 2,6-alkoxyphenyl)phosphine and its tetrafluoroborate is stable in the air. Compared with known synthetic routes of ligands used in activating carbon-chlorine bonds, the method of current invention is short, easy to operate. Moreover, with this type of ligands, the Suzuki coupling products of optically active chlorolactones and arylboronic acids would maintain their configuration and optical purity.
A new, readily available monophosphine tetrafluoroborate salt [L2·HBF4] was developed for the palladium-catalyzed amination reaction of aryl chlorides in moderate to high yields with the cheap and easily available potassium hydroxide as the base. The reaction enjoys a wide scope, lower reaction temperatures, shorter reaction times, high yields, and low catalyst loading when compared to some of same amination reactions reported in the literature. Based on a kinetic study, 31P NMR measurements, and DFT calculations, a mechanism involving a 1:1 Pd/L species is proposed.
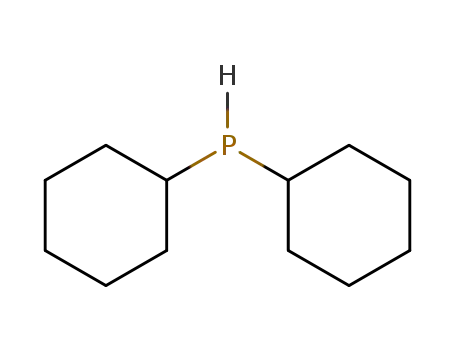
dicyclohexylphosphane

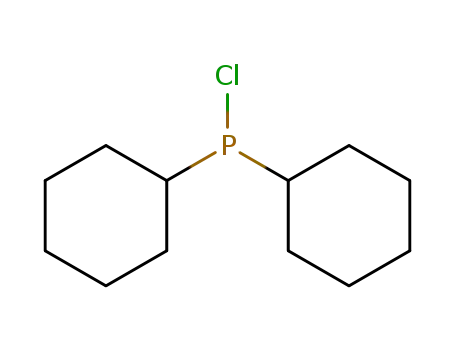
chlorodicyclohexylphosphane
| Conditions | Yield |
|---|---|
|
With
Ethyl trichloroacetate;
In
chlorobenzene;
at 80 ℃;
for 2.46667h;
Product distribution / selectivity;
|
80.6% |
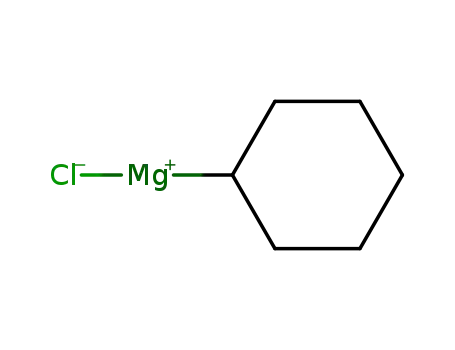
cyclohexylmagnesiumchloride


chlorodicyclohexylphosphane
| Conditions | Yield |
|---|---|
|
With
phosphorus trichloride;
|
|
|
With
phosphorus trichloride;
In
diethyl ether;
|
|
|
With
phosphorus trichloride;
In
toluene;
at 0 ℃;
|
|
|
With
phosphorus trichloride;
In
diethyl ether;
at -40 - 20 ℃;
|
|
|
Multi-step reaction with 3 steps
2: HCl
With
hydrogenchloride;
|
|
|
With
phosphorus trichloride;
In
diethyl ether;
at -40 - 30 ℃;
|
|
|
With
phosphorus trichloride;
In
diethyl ether;
at -40 - 20 ℃;
Product distribution / selectivity;
|
|
|
With
phosphorus trichloride;
In
tetrahydrofuran;
at -40 - 20 ℃;
for 7h;
|
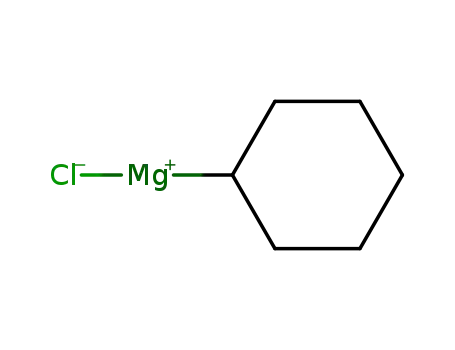
cyclohexylmagnesiumchloride
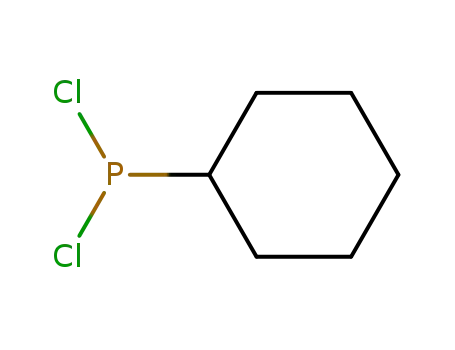
dichlorocyclohexylphosphine
cyclohexane
1-bromocyclohexane
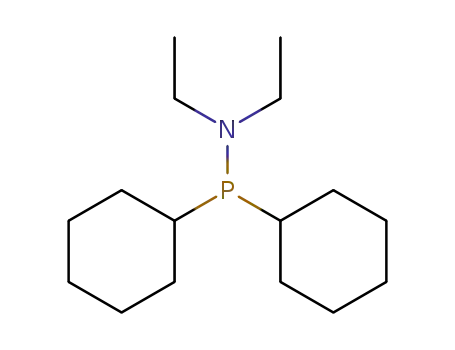
diethyl-dicyclohexylphosphino-amine
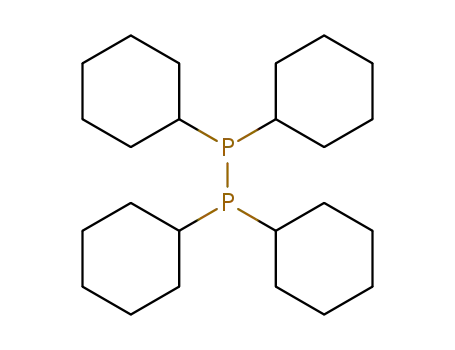
tetracyclohexyldiphosphine
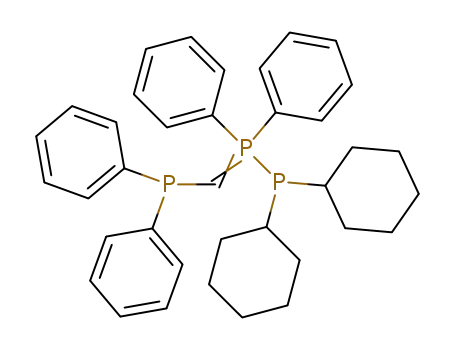
C37H43P3
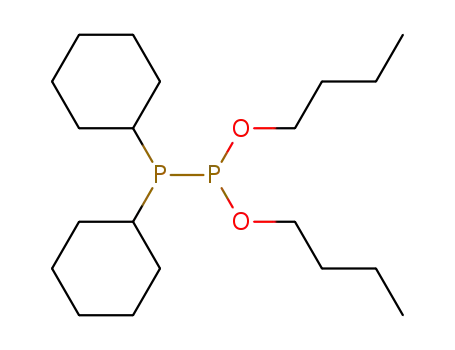
1.1-Dibutoxy-2.2-dicyclohexyldiphosphin
CAS:1055888-89-5
CAS:6372-42-5