Your Location:Home >Products >Organic phosphines >Tert-butyl phosphines >1055888-89-5
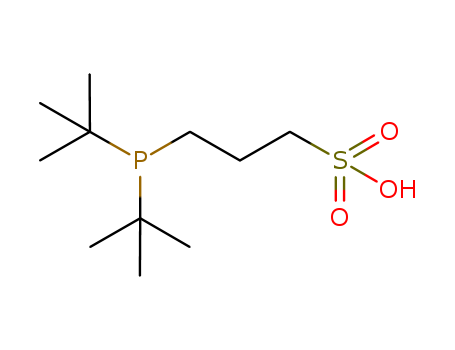

Product Details
Uses
Air stable, water soluble phosphine ligand used in palladium catalyzed cross-coupling reactions
Fluorescence spectroscopy is a powerful, extremely sensitive technique for the investigation of enzyme and ribozyme mechanisms. Herein, we describe the synthesis and characterization of water-soluble fluorescence probes for studying biocatalytic Diels-Alder reactions. These probes consist of anthracene and sulfonated BODIPY fluorophores fused by conjugated phenylacetylenyl bridges. Intact anthracene efficiently quenches BODIPY fluorescence, likely by photoinduced electron transfer. Upon destruction of the aromatic system by the Diels-Alder reaction, the fluorescence emission increases 20-fold. Binding in the catalytic pocket of a Diels-Alderase ribozyme yields a further ~2-fold increase in the fluorescence intensity of both the anthracene-BODIPY and the Diels-Alder-product-BODIPY probes. Therefore, a fluorescence-based distinction of free substrate, bound substrate, bound product, and free product is possible. With these all-in-one reporters, we monitored RNA-catalyzed Diels-Alder reactions under both single- and multiple-turnover conditions down to the nanomolar concentration range. Burst analysis at the single-molecule level revealed blinking of the dyads between an on state and an off state, presumably due to rotation around the phenylacetylenyl bridge. Binding to the ribozyme does not increase the intensity of the individual fluorescence bursts, but rather increases the average time spent in the on state. Variations in the quantum yields of the different probes correlate well with the degree of conjugation between anthracene and the phenylacetylenyl bridge.
3-(Di-tert-butylphosphonium)propane sulfonate (DTB-PPS) and 3-(diadamantylphosphonium)propane sulfonate (DAPPS) are air-stable pre-ligands for aqueous-phase palladium-catalyzed cross-coupling reactions. Both DTBPPS and DAPPS were found to give active catalysts for the Sonogashira coupling of aryl bromides at room temperature and 4-chloroanisole at 80 °C. These ligands also gave effective catalysts for the aqueous-phase Suzuki coupling of aryl bromides at room temperature.
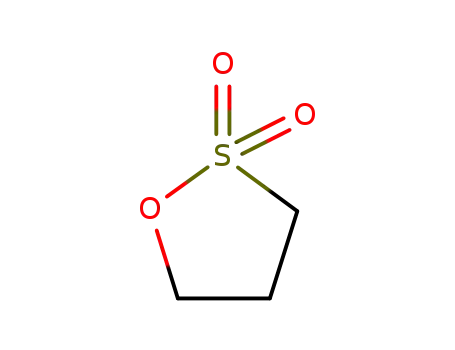
1,3-propanesultone

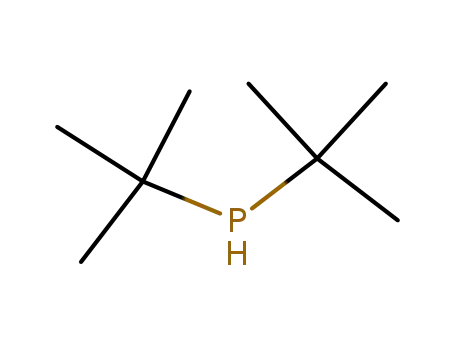
di-tert-butylphosphine

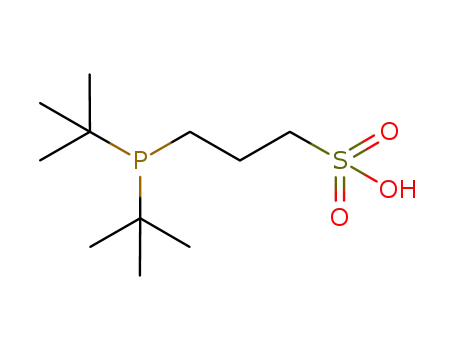
3-(di-tert-butylphosphino)-propane-1-sulfonic acid
| Conditions | Yield |
|---|---|
|
In
1,4-dioxane;
for 12h;
Inert atmosphere;
Reflux;
|
81% |
|
In
1,4-dioxane;
for 12h;
Inert atmosphere;
Reflux;
|

1,3-propanesultone

di-tert-butylphosphine
CAS:3172-56-3
CAS:119269-24-8
CAS:479094-62-7
CAS:16523-54-9