Your Location:Home >Products >Organic phosphines >Tert-butyl phosphines >1160861-53-9
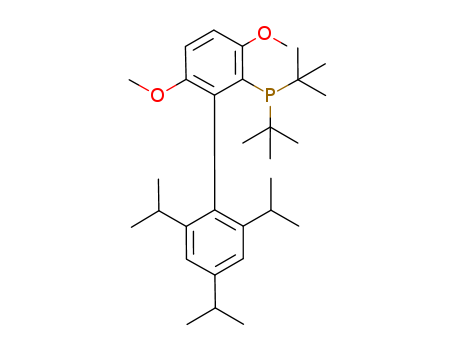

Product Details
Reaction
Ligand used in the Pd-catalyzed conversion of aryl and vinyl triflates to bromides and chlorides. Ligand used in the Pd-catalyzed O-arylation of ethyl acetohydroximates. Ligand used in the Pd-catalyzed conversion of aryl chlorides, triflates, and nonaflates to nitroaromatics. Ligand used in the Pd-catalyzed cross-coupling of amides and aryl mesylates.
Uses
Buchwald Phosphine Ligands for chemical Synthesis
General Description
tBuBrettPhos is a dialkylbiaryl phosphine ligand developed by the Buchwald group. It promotes cross-coupling reactions more efficiently and exhibits improved reactivity compared to other catalytic systems.
The invention discloses a method for synthesizing a large-steric-hindrance biphenyl organic phosphine compound, relates to a synthesis method of 2-dialkylphosphine-3,6-dimethoxy-2',4',6'-triisopropyl-1,1'-biphenyl, and belongs to the field of organic synthesis. In a water-free oxygen-free atmosphere, dialkyl phosphine chloride is used as a raw material to react with magnesium to generate dialkyl phosphine magnesium chloride, and then dialkyl phosphine magnesium chloride reacts with 2-halo-3,6-dimethoxy-2',4',6'-triisopropyl-1,1'-biphenyl under the action of a nickel catalyst to generate 2-dialkylphosphine-3,6-dimethoxy-2',4',6'-triisopropyl-1,1'-biphenyl. Compared with the prior art, the method has the advantages of mild reaction conditions, high yield and simple post-treatment, and is suitable for industrial production.
Improved processes for the preparation of biphenyl-based phosphine ligands t-BuBrettPhos, RockPhos, and BrettPhos are presented. The new methods, featuring the use of Grignard reagents and catalytic amounts of copper, are superior to the previous methods, which require the use of tert-butyllithium and stoichiometric amounts of copper. Specifically, the use of less dangerous reagents provides a safer process, while the use of catalytic amounts of copper allows for the isolation of pure products in high yield. These improvements are particularly significant for the large-scale preparation of these ligands. Copyright
A catalyst based on a new biarylphosphine ligand (3) for the Pd-catalyzed cross-coupling reactions of amides and aryl chlorides is described. This system shows the highest turnover frequencies reported to date for these reactions, especially for aryl chloride substrates bearing an ortho substituent. An array of amides and aryl chlorides were successfully reacted in good to excellent yields.
Ligands for transition metals are disclosed herein, which may be used in various transition-metal-catalyzed carbon-heteroatom and carbon-carbon bond-forming reactions. The disclosed methods provide improvements in many features of the transition-metal-catalyzed reactions, including the range of suitable substrates, number of catalyst turnovers, reaction conditions, and efficiency. For example, improvements have been realized in transition-metal-catalyzed cross-coupling reactions.
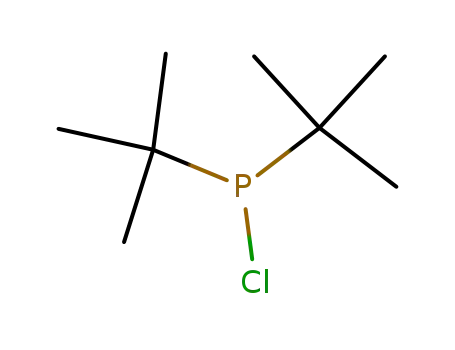
di(tert-butyl)chlorophosphine

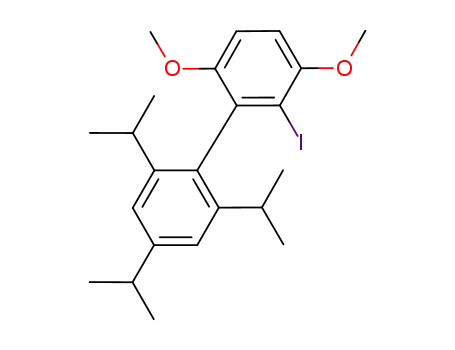
2-iodo-2’,4’,6’-triisopropyl-3,6-dimethoxybiphenyl

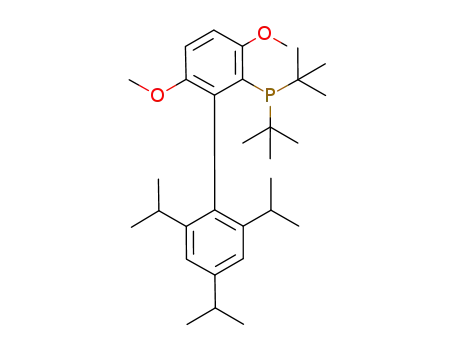
t-BuBrettPhos
| Conditions | Yield |
|---|---|
|
di(tert-butyl)chlorophosphine;
With
magnesium;
In
tetrahydrofuran;
at -10 - 10 ℃;
for 2h;
Inert atmosphere;
2-iodo-2’,4’,6’-triisopropyl-3,6-dimethoxybiphenyl;
With
tetrakis(triphenylphosphine)nickel(0);
In
tetrahydrofuran;
at 80 ℃;
for 12h;
|
92% |
|
2-iodo-2’,4’,6’-triisopropyl-3,6-dimethoxybiphenyl;
With
tert.-butyl lithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 0.833333h;
Inert atmosphere;
di(tert-butyl)chlorophosphine;
With
copper(l) chloride;
In
tetrahydrofuran; hexane;
at -78 - 70 ℃;
for 60.1667h;
Inert atmosphere;
|
53% |

di(tert-butyl)chlorophosphine

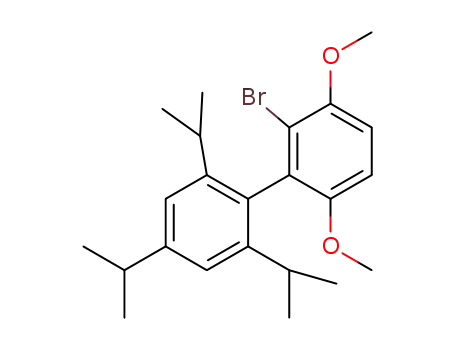
2-bromo-3,6-dimethoxy-2',4',6'-triisopropyl-1,1'-biphenyl


t-BuBrettPhos
| Conditions | Yield |
|---|---|
|
di(tert-butyl)chlorophosphine;
With
magnesium;
In
tetrahydrofuran;
at -10 - 10 ℃;
for 2h;
Inert atmosphere;
2-bromo-3,6-dimethoxy-2',4',6'-triisopropyl-1,1'-biphenyl;
With
bis(acetylacetonate)nickel(II);
In
tetrahydrofuran;
at 80 ℃;
for 12h;
|
90% |
|
2-bromo-3,6-dimethoxy-2',4',6'-triisopropyl-1,1'-biphenyl;
With
ethylene dibromide;
In
tetrahydrofuran; toluene;
at 80 ℃;
for 3h;
Inert atmosphere;
di(tert-butyl)chlorophosphine;
With
copper(l) chloride; lithium bromide;
In
tetrahydrofuran; toluene;
at 120 ℃;
for 20h;
Inert atmosphere;
|
87% |

di(tert-butyl)chlorophosphine
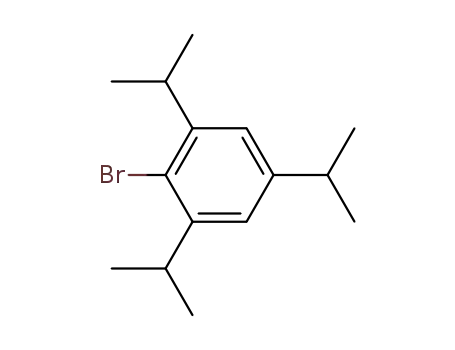
2,4,6-triisopropyl-1-bromobenzene
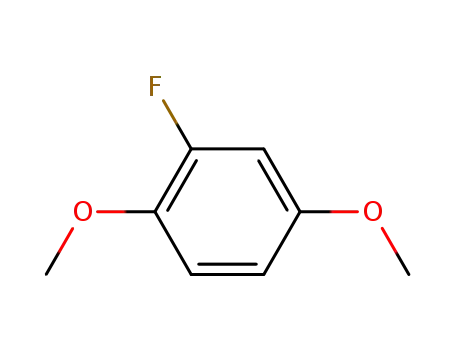
2-fluoro-1,4-dimethoxybenzene

2-iodo-2’,4’,6’-triisopropyl-3,6-dimethoxybiphenyl
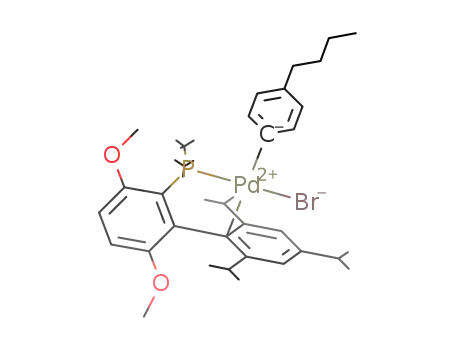
PdBrC6H4(CH2)3CH3C6H2(CH(CH3)2)3C6H2(OCH3)2P(C(CH3)3)2
CAS:1019-71-2
CAS:819-19-2
CAS:479094-62-7