Your Location:Home >Products >Organic phosphines >Phenyl phosphines >41593-58-2
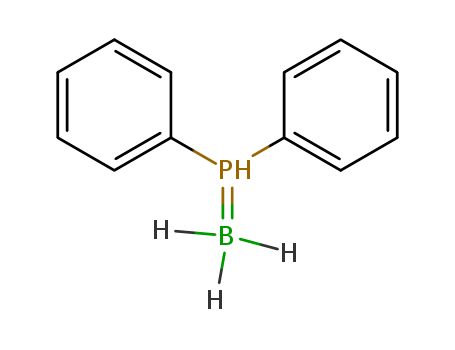

Product Details
Chemical Properties
white to off-white powder or crystals
Uses
Reactant for:Nucleophilic addition reactions (anion)Catalytic dehydrogenation in the presence of ruthenium bidentate phosphine compelxesDeprotonation reactionsCatalytic coupling with alkynyl bromidesCatalyst-free Staudinger ligation for indirect 18F-radiolabelingPreparation of chiral phosphine-phosphite bidentate ligands with biphenyl backboneReactions with frustrated lewis pair combinations of group 14 triflates and sterically hindered nitrogen bases
A Ni/(S,S)-BDPP-catalyzed intramolecular Heck cyclization of N-(2-iodo-aryl) acrylamide with 2-methyl-2-phenylmalononitrile was developed to give oxindoles with good enantioselectivities. We found that utilizing such an electrophilic cyanation reagent cou
Reduction of phosphine oxides into the corresponding phosphines using PhSiH3 as a reducing agent and Ph3C+[B(C6F5)4]? as an initiator is described. The process is highly efficient, reducing a broad range of secondary and tertiary alkyl and arylphosphines, bearing various functional groups in generally good yields. The reaction is believed to proceed through the generation of a silyl cation, which reaction with the phosphine oxide provides a phosphonium salt, further reduced by the silane to afford the desired phosphine along with siloxanes. (Figure presented.).
A highly versatile synthesis of amine-boranes via carbonyl reduction by sodium borohydride is described. Unlike the prior bicarbonate-mediated protocol, which proceeds via a salt metathesis reaction, the carbon dioxide-mediated synthesis proceeds via reduction to a monoformatoborohydride intermediate. This has been verified by spectroscopic analysis, and by using aldehydes and ketones as the carbonyl source for the activation of sodium borohydride. This process has been used to produce borane complexes with 1°-, 2°-, and 3°-amines, including those with borane reactive functionalities, heteroarylamines, and a series of phosphines.
An effective and mild synthetic method for secondary phosphine-boranes from secondary phosphine oxides was developed. The slow addition of borane-tetrahydrofuran to a solution of secondary phosphine oxides at ambient temperature gave secondary phosphine-boranes with high yield. The use of air-sensitive secondary phosphines, which are common substrates for secondary phosphine-borane syntheses, is avoided in this mild process. Moreover, generation of by-products, such as secondary hydroxy-phosphine-boranes, is also minimized.
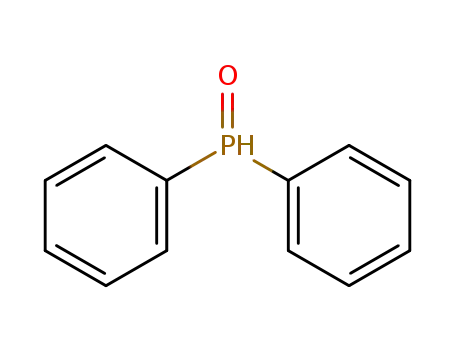
Diphenylphosphine oxide

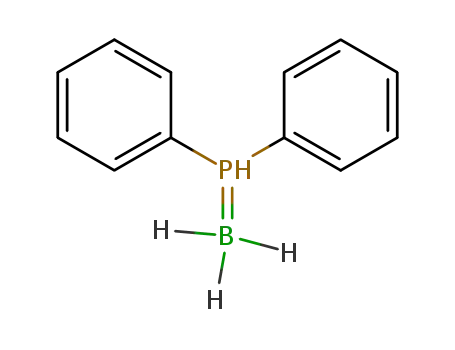
diphenylphosphineborane
| Conditions | Yield |
|---|---|
|
With
borane-THF;
In
tetrahydrofuran; toluene;
at 25 ℃;
|
70.3% |
|
Multi-step reaction with 2 steps
1: titanium(IV) isopropylate; 1,1,3,3-Tetramethyldisiloxane / toluene / 60 °C / Inert atmosphere
2: borane-THF / toluene / 0 - 20 °C
With
titanium(IV) isopropylate; borane-THF; 1,1,3,3-Tetramethyldisiloxane;
In
toluene;
|
|
|
With
borane-THF;
In
toluene;
|
|
|
Multi-step reaction with 2 steps
1: indium(III) bromide; 1,1,3,3-Tetramethyldisiloxane / toluene / 18 h / 100 °C / Inert atmosphere; Sealed tube
2: dimethylsulfide borane complex / tetrahydrofuran; toluene / 1 h / 0 - 20 °C / Inert atmosphere
With
indium(III) bromide; 1,1,3,3-Tetramethyldisiloxane; dimethylsulfide borane complex;
In
tetrahydrofuran; toluene;
|
|
|
Multi-step reaction with 2 steps
1: diisobutylaluminium hydride / dichloromethane / 0.33 h / 0 °C / Inert atmosphere
2: tetrahydrofuran / 2 h / Inert atmosphere
With
diisobutylaluminium hydride;
In
tetrahydrofuran; dichloromethane;
|
|
|
Multi-step reaction with 2 steps
1.1: trityl tetrakis(pentafluorophenyl)borate / (2)H8-toluene / 20 °C / Glovebox; Inert atmosphere
1.2: 80 °C / Glovebox; Inert atmosphere; Sealed tube
2.1: (2)H8-toluene / 12 h / 20 °C / Glovebox; Inert atmosphere
With
trityl tetrakis(pentafluorophenyl)borate;
In
(2)H8-toluene;
|
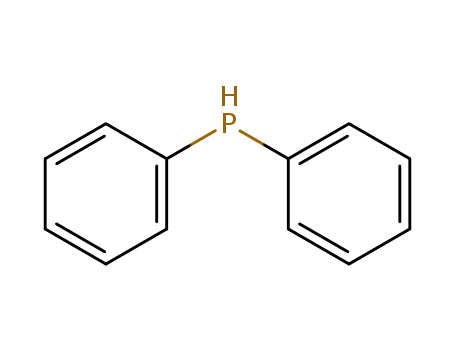
diphenylphosphane


diphenylphosphineborane
| Conditions | Yield |
|---|---|
|
With
borane-THF;
In
tetrahydrofuran;
for 7h;
Inert atmosphere;
|
99% |
|
diphenylphosphane;
With
dimethylsulfide borane complex;
In
dichloromethane;
at 20 ℃;
Inert atmosphere;
With
ammonium chloride;
In
dichloromethane; water;
|
97% |
|
With
sodium tetrahydroborate; acetic acid;
In
tetrahydrofuran;
at 0 - 20 ℃;
|
95% |
|
With
borane-THF;
at 20 ℃;
Cooling with ice;
|
89% |
|
With
dimethylsulfide borane complex;
In
toluene;
at 23 ℃;
|
|
|
With
borane-THF;
In
tetrahydrofuran;
at -78 ℃;
Inert atmosphere;
|
|
|
With
borane-THF;
In
toluene;
at 0 - 20 ℃;
|
|
|
With
borane-THF;
In
tetrahydrofuran;
at 0 - 20 ℃;
for 2.08333h;
Inert atmosphere;
|
|
|
With
dimethylsulfide borane complex;
In
tetrahydrofuran; toluene;
at 0 - 20 ℃;
for 1h;
Inert atmosphere;
|
|
|
With
dimethylsulfide borane complex;
In
tetrahydrofuran;
Inert atmosphere;
|

diphenylphosphane

Diphenylphosphine oxide
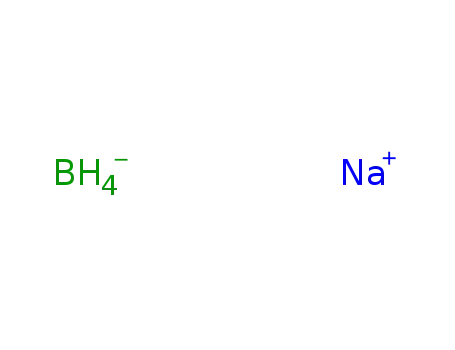
sodium tetrahydroborate
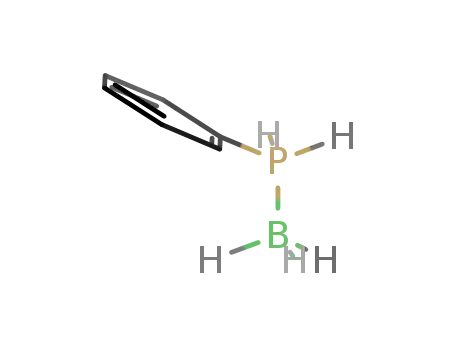
phenylphosphine-borane
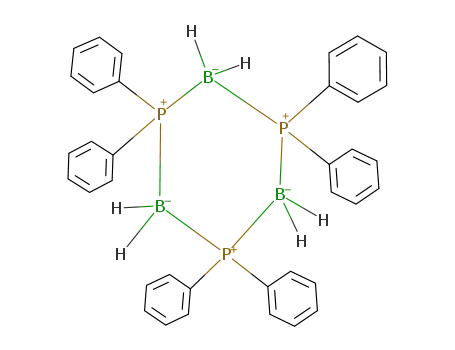
(Diphenylphosphino)borane
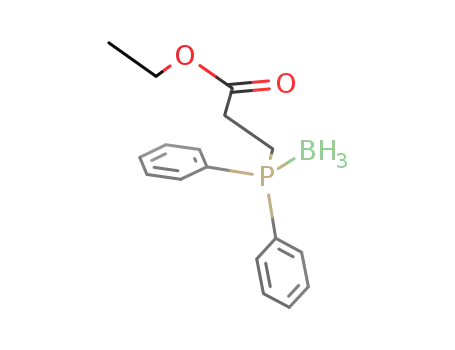
(C6H5)2P(BH3)CH2CH2CO2C2H5
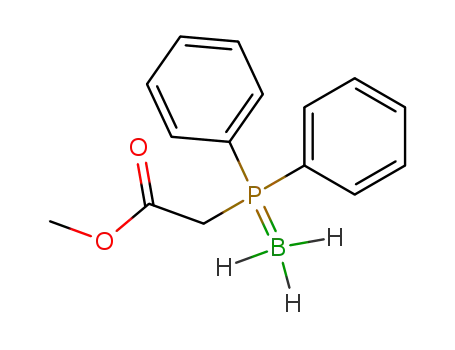
(C6H5)2P(BH3)CH2CO2CH3
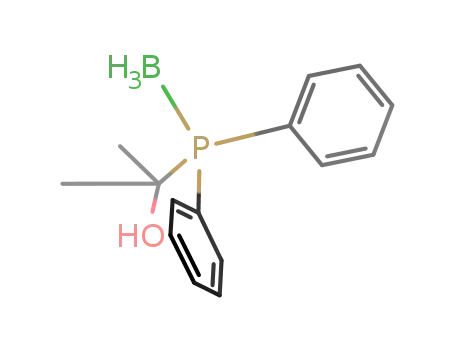
(C6H5)2P(BH3)C(OH)(CH3)2
CAS:1019-71-2
CAS:30540-36-4