Your Location:Home >Products >Organic phosphines >Phenyl phosphines >30540-36-4


Product Details
A series of novel phospholium amphiphilic compounds with straight alkyl chains with different numbers of carbon atoms (12, 14, 15, 16, 17, 18) were synthesized. The quaternary phosphorus, phosphonium cation, is incorporated into a five-membered heterocyclic ring. Their physicochemical properties were investigated by measurements of surface tension, conductivity and dynamic light scattering. The critical micelle concentration (cM), the surface tension value at the cM (γcmc), the surface area at the surface saturation per head group (Acmc), the ionization degree of micelle (α), the free energy of micellization (ΔG°mic), and hydrodynamic diameter (dh) were determined. Antimicrobial activity was tested against bacteria and yeast. The structure–activity relationship was determined.
Unprecedented formal transition-metal-catalyzed phosphole C-H functionalization is described in this paper. Pentasubstituted phospholes were prepared via the copper-catalyzed reaction of 1,3,4-trisubstituted phosphole with aryl iodides or bromides under distinct conditions. The developed methodology is able to accommodate a wide variety of substituents, including aryl, heteroaryl, and alkenyl.
The synthesis of 3,4-dimethyl-1-phenylphosphole, 3,4-dimethyl-1-phenyl-λ5-phosphole 1-oxide dimer, and 3,4-dimethyl-1-phenyl-2,5-dihydrophosphole 1-oxide is described.
The chemical compound 3,4-dimethyl-2,5,6-tris(p-sulfonatophenyl)-1-phosphanorbernadiene, a process for the preparation thereof and a process for the hydroformylation of olefinically unsaturated compounds with the use of catalysts which contain the aforeme
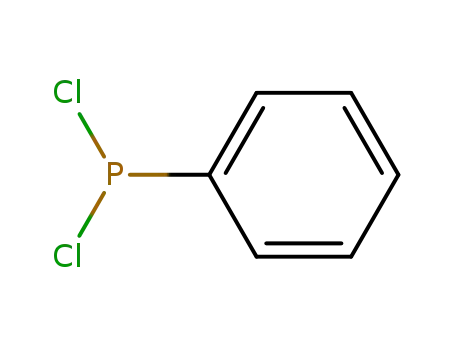
Dichlorophenylphosphine

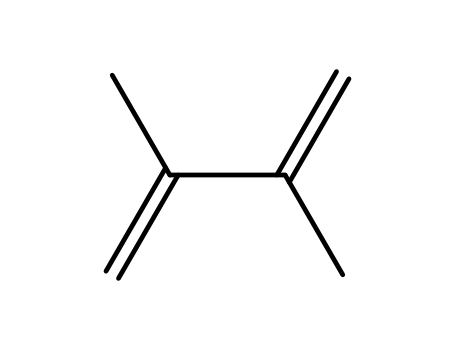
2,3-dimethyl-buta-1,3-diene


3,4-dimethyl-1-phenylphosphole
| Conditions | Yield |
|---|---|
|
Dichlorophenylphosphine; 2,3-dimethyl-buta-1,3-diene;
at 20 ℃;
for 168h;
Inert atmosphere;
With
α-picoline;
at 20 ℃;
for 48h;
Inert atmosphere;
|
50% |
|
With
α-picoline;
|
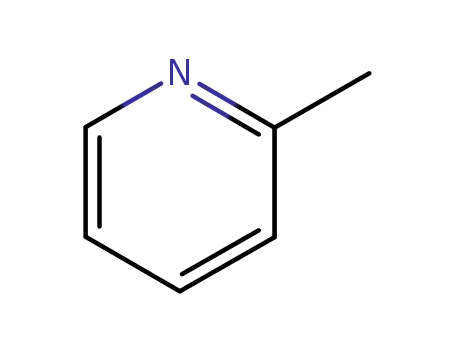
α-picoline

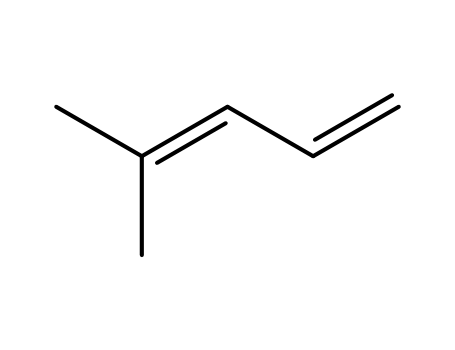
4-methyl-1,3-pentadiene


Dichlorophenylphosphine

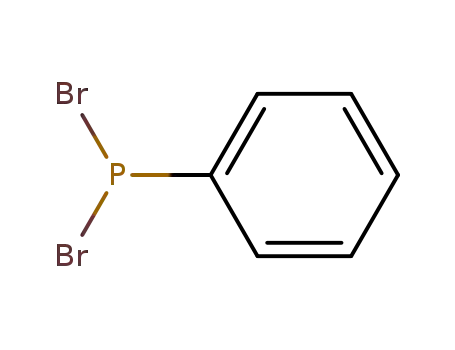
phenyldibromophosphine


3,4-dimethyl-1-phenylphosphole
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
hexane; dichloromethane; nitrogen; water;
|

2,3-dimethyl-buta-1,3-diene

phenyldibromophosphine

Dichlorophenylphosphine

α-picoline
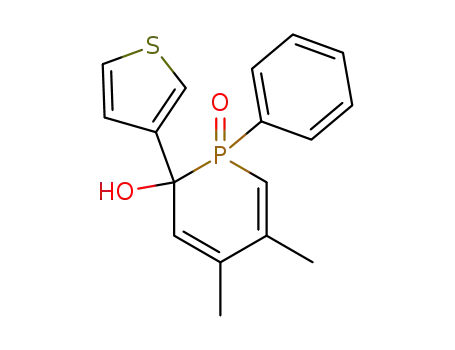
4,5-dimethyl-1-oxo-1-phenyl-2-thiophen-3-yl-1,2-dihydro-1λ5-phosphinin-2-ol
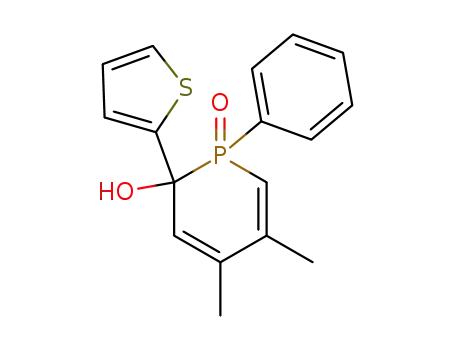
oxyde de phenyl-1-(thienyl-2')-2-hydroxy-2-dimethyl-4,5-dihydro-1,2-phosphorine
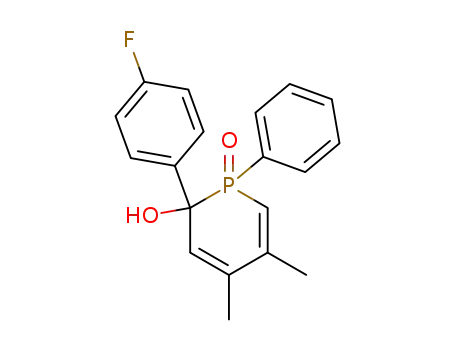
2-(4-fluoro-phenyl)-4,5-dimethyl-1-oxo-1-phenyl-1,2-dihydro-1λ5-phosphinin-2-ol
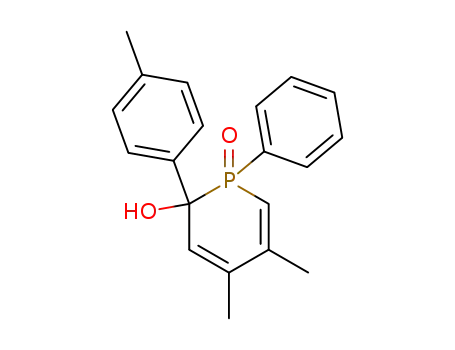
4,5-dimethyl-1-oxo-1-phenyl-2-p-tolyl-1,2-dihydro-1λ5-phosphinin-2-ol
CAS:1448787-63-0
CAS:41593-58-2
CAS:1019-71-2