Your Location:Home >Products >Organic phosphines >Phenyl phosphines >1019-71-2
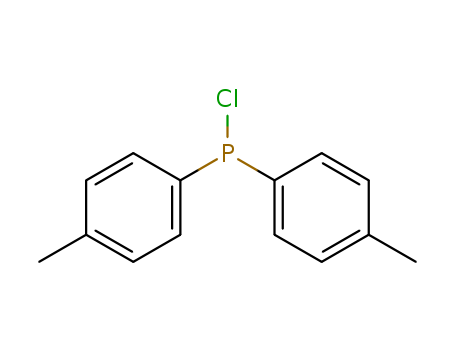

Product Details
Uses
Chlorodi(p-tolyl)phosphine is used as a reactant for synthesis of palladium catalysts for C-C bond forming cross-coupling reactions, ligands for nickel(0)-catalyzed isomerization reactions, phosphinosulfonamide nickel complexes for oligomerization reactions. It is a reactant for phosphinylation reactions.
Uses
Reactant for synthesis of:Palladium catalysts for C-C bond forming cross-coupling reactionsLigands for nickel(0)-catalyzed isomerization reactionsPhosphinosulfonamide nickel complexes for oligomerization reactionsReactant for:Pd-catalyzed intramolecular asymmetric allylic amination and ring-closing metathesisPhosphinylation reactions
InChI:InChI=1/C14H14ClP/c1-11-3-7-13(8-4-11)16(15)14-9-5-12(2)6-10-14/h3-10H,1-2H3
C?H bond activation has been established as an attractive strategy to access axially chiral biaryls, and the most straightforward method is direct C?H arylation of arenes. However, the arylating source has been limited to several classes of reactive and bulky reagents. Reported herein is rhodium-catalyzed 1:2 coupling of diarylphosphinic amides and diarylacetylenes for enantio- and diastereoselective construction of biaryls with both central and axial chirality. This twofold C?H activation reaction stays contrast to the previously explored Miura–Satoh type 1:2 coupling of arenes and alkynes in terms of chemoselectivity and proceeded under mild conditions with the alkyne acting as a rare arylating reagent. Both C?H activation events are stereo-determining and are under catalyst control, with the 2nd C?H activation being diastereo-determining in a remote fashion. Analysis of the stereochemistry of the major and side products suggests moderate enantioselectivity of the initial C?H activation–desymmetrization process. However, the minor (R) rhodium vinyl intermediate is consumed more readily in undesired protonolysis, eventually resulting in high enantio- and diastereoselectivity of the major product.
Polydentate ligands having both soft and hard centers are very effective ligands for the preparation of transition metal complexes. PNNP-type tetradentate diaminodiphosphine ligands are most preferred ligand types cause of their good efficiency for the asymmetric reactions. In this study, three different iminophosphine derivative PNNP-type chiral ligands were synthesized using (R)-(+)-1,1′-Binaphthyl-2,2′-diamine (R-BINAM) and d?phenylphosph?no benzaldehyde derivatives.
A ruthenium/C3-TunePhos catalytic system has been identified for highly efficient direct reductive amination of simple ketones. The strategy makes use of ammonium acetate as the amine source and H2 as the reductant and is a user-friendly and operatively simple access to industrially relevant primary amines. Excellent enantiocontrol (>90% ee for most cases) was achieved with a wide range of alkyl aryl ketones. The practicability of this methodology has been highlighted by scalable synthesis of key intermediates of three drug molecules. Moreover, an improved synthetic route to the optimal diphosphine ligand C3-TunePhos is also presented.
The invention discloses a method for preparing a diarylphosphochlorine compound and belongs to the field of organic synthesis. The method is as follows: the diarylphosphochlorine compound is preparedby reaction of triarylphosphine as a starting material with phosphorus trichloride in the presence of zinc trifluoromethanesulfonate as a catalyst and distillation. Compared with the prior art, the method has the advantages of high reaction yield and simple post-treatment, is particularly suitable for preparation of the diarylphosphochlorine compound with a substituent, and is more suitable for industrial production. The obtained diarylphosphochlorine compound can be used as a ligand for synthesizing metal catalysts, and is applied to the fields such as organic photoelectric materials and medicines.
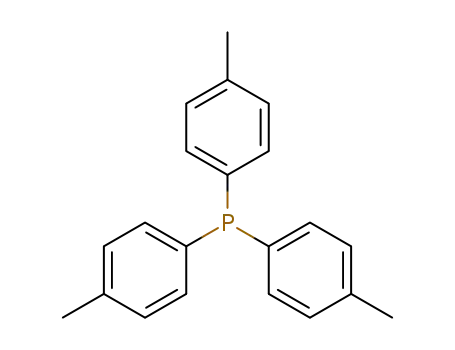
Tri(p-tolyl)phosphine

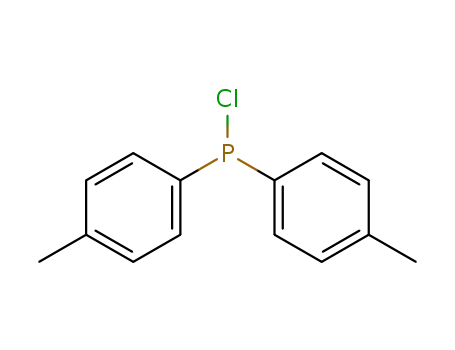
chlorodi-p-tolylphosphane
| Conditions | Yield |
|---|---|
|
With
zinc trifluoromethanesulfonate; phosphorus trichloride;
In
tetrachloromethane;
at 0 - 60 ℃;
for 12h;
|
91% |
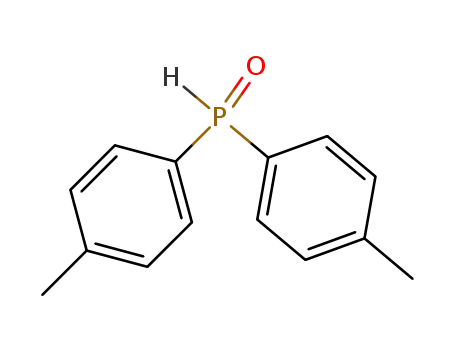
di(p-tolyl)phosphine oxide


chlorodi-p-tolylphosphane
| Conditions | Yield |
|---|---|
|
With
phosphorus trichloride;
In
toluene; benzene;
at 0 - 10 ℃;
for 0.5h;
|
65% |
|
With
phosphorus trichloride;
In
dichloromethane;
at 20 ℃;
for 2h;
Inert atmosphere;
Schlenk technique;
|
|
|
With
phosphorus trichloride;
In
toluene;
at 20 ℃;
for 1h;
Inert atmosphere;
|
|
|
With
phosphorus trichloride;
In
benzene;
at 0 ℃;
Inert atmosphere;
Glovebox;
Heating;
|
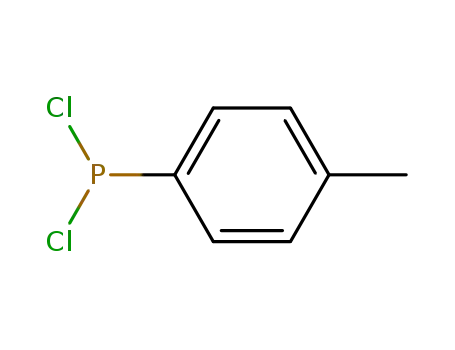
p-tolyldichlorophosphine
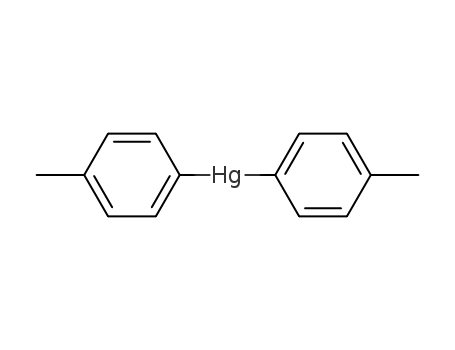
bis(p-tolyl)mercury
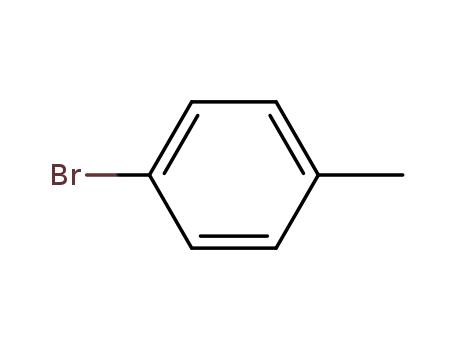
para-bromotoluene
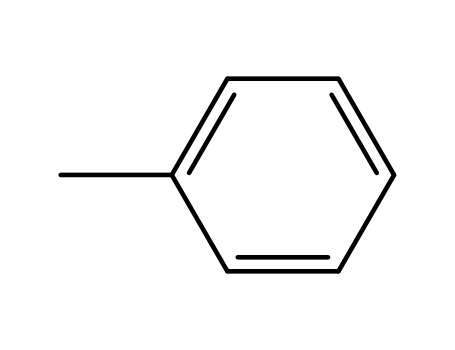
toluene
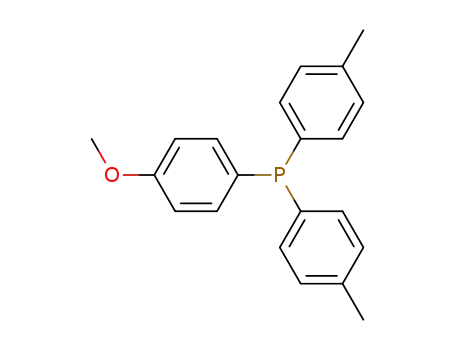
Bis-(p-tolyl)-p-anisyl-phosphin

di-p-tolylphosphine
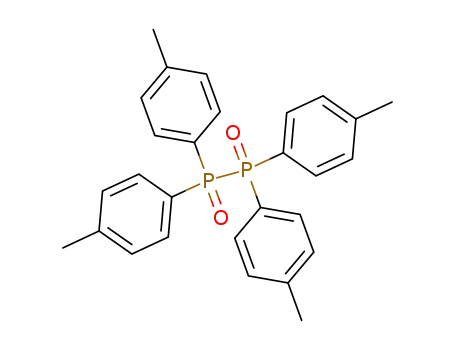
Tetra-

1-(di-p-tolylphosphino)-2,3,4,5-tetramethylcyclopentadiene
CAS:13885-09-1
CAS:76186-72-6
CAS:30540-36-4
CAS:21406-61-1