Your Location:Home >Products >Organic phosphines >Phenyl phosphines >21406-61-1
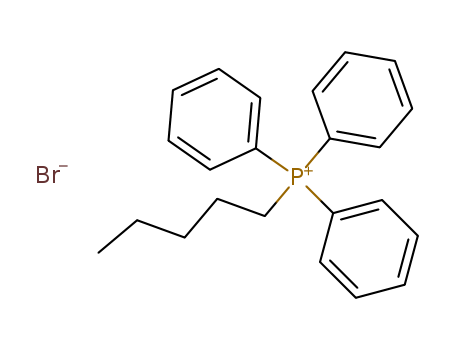
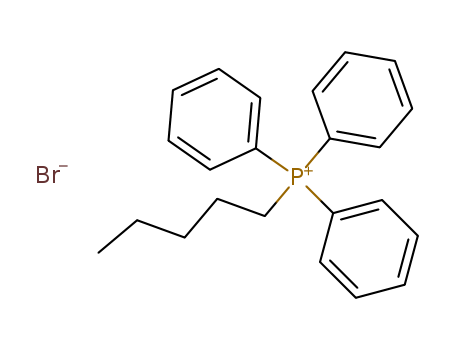
Product Details
Chemical Properties
LIGHT BROWN TO YELLOW-BROWN CRYSTALLINE POWDER
Uses
It is used as pharmaceutical intermediates.
InChI:InChI=1/C23H26P.BrH/c1-2-3-13-20-24(21-14-7-4-8-15-21,22-16-9-5-10-17-22)23-18-11-6-12-19-23;/h4-12,14-19H,2-3,13,20H2,1H3;1H/q+1;/p-1
The present invention provide for preparing a formylalkenyl alkoxymethyl ether compound of the following general formula (2): R3CH2OCH2O(CH2)aCH═CHCHO (2), wherein R3 represents a hydrogen atom, an n-alkyl group having 1 to 9 carbon atoms, or a phenyl group; and “a” represents an integer of 1 to 10, the process comprising: hydrolyzing a dialkoxyalkenyl alkoxymethyl ether compound of the following general formula (1): R3CH2OCH2O(CH2)aCH═CHCH(OR1)(OR2) (1), wherein R1 and R2 represent, independently of each other, a monovalent hydrocarbon group having 1 to 15 carbon atoms, or R1 and R2 may form together a divalent hydrocarbon group, R1-R2, having 2 to 10 carbon atoms; and R3 and “a” are as defined above, in the presence of an acid while removing an alcohol compound thus generated to form the formylalkenyl alkoxymethyl ether compound (2).
Interactions on the molecular level control structure as well as function. Especially interfaces between innocent alkyl groups are hardly studied although they are of great importance in larger systems. Herein, London dispersion in conjunction with solvent interactions between linear alkyl chains was examined with an azobenzene-based experimental setup. Alkyl chains in all meta positions of the azobenzene core were systematically elongated, and the change in rate for the thermally induced Z→E isomerization in n-decane was determined. The stability of the Z-isomer increased with longer chains and reached a maximum for n-butyl groups. Further elongation led to faster isomerization. The origin of the intramolecular interactions was elaborated by various techniques, including 1H NOESY NMR spectroscopy. The results indicate that there are additional long-range interactions between n-alkyl chains with the opposite phenyl core in the Z-state. These interactions are most likely dominated by attractive London dispersion. This work provides rare insight into the stabilizing contributions of highly flexible groups in an intra- as well as an intermolecular setting.
A process to prepare (9E,11Z)-9,11-hexadecadienyl acetate with a good yield and high purity of the general formula (1): CH3—(CH2)3—CH═CH—CH═CH—(CH2)a—X.=The process includes a step of conducting a Wittig reaction between a haloalkenal of the general formula (2): OHC—CH═CH—(CH2)a—X, and a triarylphosphonium pentylide of the general formula (3): CH3—(CH2)3—CH?—P+Ar3, to obtain the 1-haloalkadiene, and the use of a (7E,9Z)-1-halo-7,9-tetradecadiene obtained by the process for a process of preparing (9E, 11Z)-9,11-hexadecadienyl acetate.
An efficient process for preparing (9E,11Z)-9,11-hexadecadienal of formula (4) is provided. The process includes at least steps of: conducting a nucleophilic substitution reaction between an (8E,10Z)-8,10-pentadecadienyl magnesium halide derived from an (8E,10Z)-1-halo-8,10-pentadecadiene of (1): and an orthoformate ester (2) to thereby prepare a (9E, 11Z)-1,1-dialkoxy-9, 11-hexadecadiene (3): and hydrolyzing the (9E, 11Z)-1,1-dialkoxy-9,11-hexadecadiene (3) to obtain (9E, 11Z)-9,11-hexadecadienal (4).
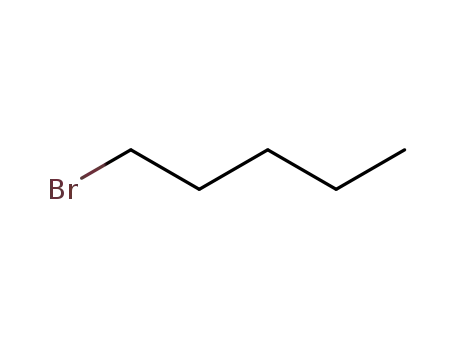
1-Bromopentane

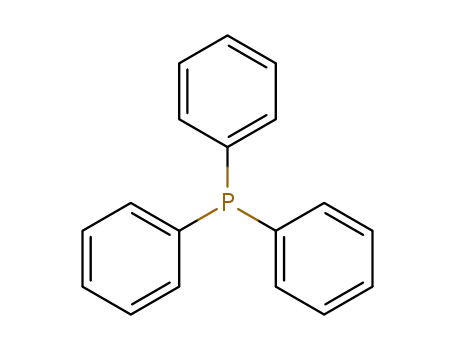
triphenylphosphine

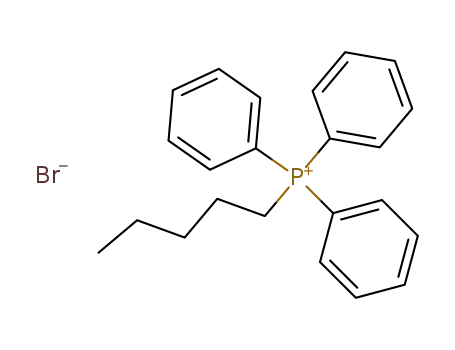
pentyltriphenylphosphonium bromide
| Conditions | Yield |
|---|---|
|
In
toluene;
Heating;
|
95% |
|
for 18h;
Heating;
|
94.4% |
|
In
toluene;
Reflux;
|
93% |
|
In
acetonitrile;
for 24h;
Heating;
|
92% |
|
In
acetonitrile;
Heating;
|
92.8% |
|
In
acetonitrile;
for 24h;
Heating;
|
90% |
|
In
acetonitrile;
for 20h;
Heating;
|
90% |
|
In
acetonitrile;
for 72h;
Heating;
|
90% |
|
In
toluene;
|
86% |
|
In
toluene;
Reflux;
|
72% |
|
In
benzene;
Heating;
|
|
|
In
diethyl ether; toluene;
Heating;
|
|
|
|
|
|
In
butan-1-ol;
Yield given;
Heating;
|
|
|
In
acetonitrile; benzene;
at 90 ℃;
for 72h;
Inert atmosphere;
|
|
|
In
N,N-dimethyl-formamide;
at 110 - 115 ℃;
for 6h;
|
|
|
In
N,N-dimethyl-formamide;
at 110 - 115 ℃;
for 6h;
|
|
|
In
N,N-dimethyl-formamide;
at 110 - 120 ℃;
for 9h;
|

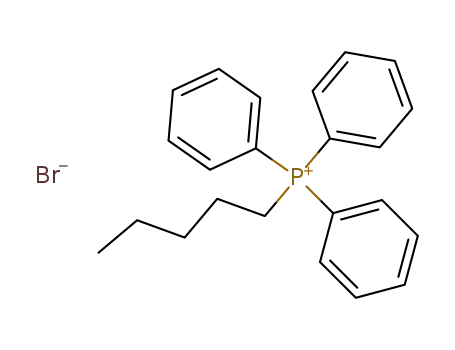
pentyltriphenylphosphonium bromide
| Conditions | Yield |
|---|---|
|
|
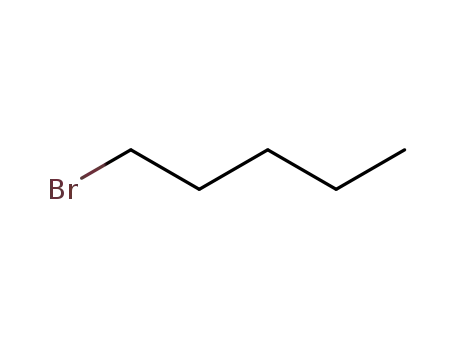
1-Bromopentane

triphenylphosphine
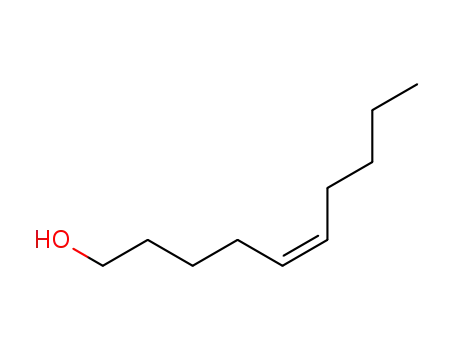
(Z)-dec-5-en-1-ol
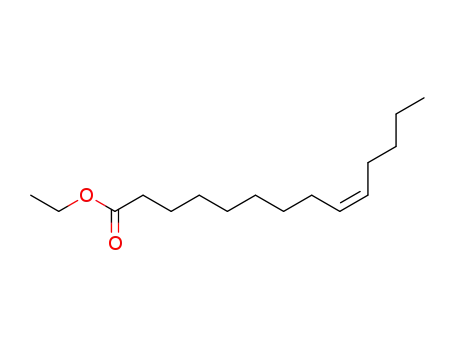
myristoleic acid ethyl ester
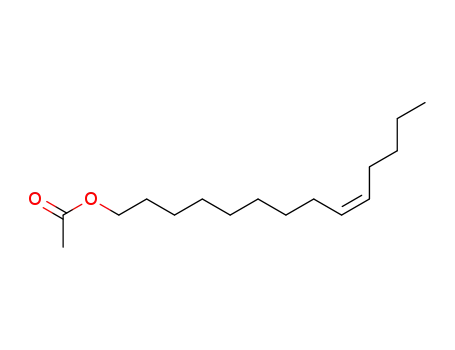
(Z)-9-tetradecen-1-yl acetate
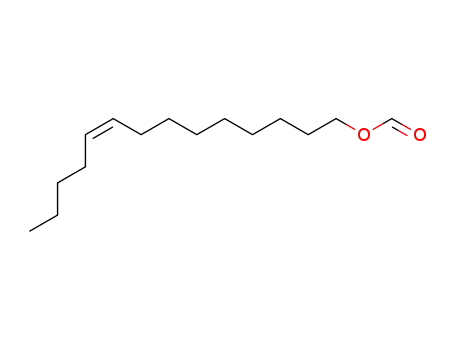
(Z)-9-tetradecen-1-ol formate
CAS:955959-91-8
CAS:1019-71-2
CAS:14647-23-5
Molecular Formula:C26H24Cl2NiP2
Molecular Weight:528