Your Location:Home >Products >OLED intermediates >Carbazoles >1357572-66-7

Product Details
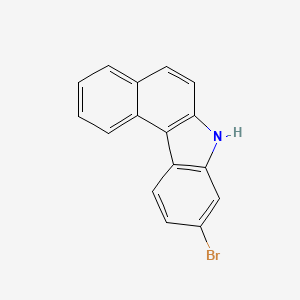
9-Bromo-7H-benzo[c]carbazole is a heterocyclic organic compound consisting of a carbazole core fused with a benzene ring, with a bromine substituent at the 9-position. This structural motif is found in various biologically active natural products and pharmaceutical compounds, making it an important scaffold in medicinal chemistry. The bromine atom provides opportunities for further functionalization through cross-coupling reactions, allowing for the synthesis of a wide range of derivatives. 9-Bromo-7H-benzo[c]carbazole has been utilized as a precursor in the preparation of luminescent materials, organic semiconductors, and as a building block in the synthesis of more complex heterocyclic systems. Puyang Huicheng Electronic Material Co., Ltd provides information about the chemical properties, structure, melting point, and boiling point of 9-bromo-7H-benzo[c]carbazole.
Isomeric SMILES: C1=CC=C2C(=C1)C=CC3=C2C4=C(N3)C=C(C=C4)Br
InChIKey: ZXRPGNSDTBITJV-UHFFFAOYSA-N
InChI: InChI=1S/C16H10BrN/c17-11-6-7-13-15(9-11)18-14-8-5-10-3-1-2-4-12(10)16(13)14/h1-9,18H
Synthsis of 9-(dibenzo[b,d]thiophen-4-yl)-7H-benzo[c]carbazole (1–3) A mixture of 9-bromo-7H-benzo[c]carbazole 1–2 (1.16 g, 4 mmol), dibenzo [b,d] thiophen-4-ylboronic acid (1.00 g, …
Synthsis of 9-(dibenzo[b,d]thiophen-4-yl)-7H-benzo[c]carbazole (1–3) A mixture of 9-bromo-7H-benzo[c]carbazole 1–2 (1.16 g, 4 mmol), dibenzo [b,d] thiophen-4-ylboronic acid (1.00 g, …
The invention relates to a compound and ...
The invention relates to an organic comp...
1-(4-bromo-2-nitrophenyl)naphthalene

9-bromo-7H-benzo[c]carbazole
| Conditions | Yield |
|---|---|
|
With triphenylphosphine; In 1,2-dichloro-benzene; at 150 ℃; for 6h;
|
64% |
|
With triphenylphosphine; In 1,2-dichloro-benzene; at 180 ℃; for 16h;
|
61.4% |
|
With triphenylphosphine; In 1,2-dichloro-benzene; for 5h; Reflux;
|
52% |
|
With triethyl phosphite; In 1,2-dichloro-benzene; at 150 ℃; for 24h;
|
37% |
|
With triethyl phosphite; In 1,2-dichloro-benzene; at 150 ℃; for 24h; Inert atmosphere;
|
37% |
|
With triethyl phosphite; at 150 ℃; for 24h;
|
1-Naphthylboronic acid

9-bromo-7H-benzo[c]carbazole
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: sodium carbonate; tetrakis(triphenylphosphine) palladium(0) / toluene; ethanol / 3 h / 90 °C
2: triethyl phosphite / 1,2-dichloro-benzene / 24 h / 150 °C
With tetrakis(triphenylphosphine) palladium(0); sodium carbonate; triethyl phosphite; In ethanol; 1,2-dichloro-benzene; toluene;
|
|
|
Multi-step reaction with 2 steps
1: tetrakis(triphenylphosphine) palladium(0); potassium carbonate / ethanol; toluene / 5 h / Reflux
2: triphenylphosphine / 1,2-dichloro-benzene / 6 h / 150 °C
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; triphenylphosphine; In ethanol; 1,2-dichloro-benzene; toluene;
|
|
|
Multi-step reaction with 2 steps
1.1: potassium carbonate / tetrahydrofuran; water / 0.25 h / Inert atmosphere
1.2: 5 h / Inert atmosphere; Reflux
2.1: triphenylphosphine / 1,2-dichloro-benzene / 5 h / Reflux
With potassium carbonate; triphenylphosphine; In tetrahydrofuran; water; 1,2-dichloro-benzene;
|
|
|
Multi-step reaction with 2 steps
1: sodium carbonate; bis-triphenylphosphine-palladium(II) chloride / toluene; ethanol / 3 h / 90 °C / Inert atmosphere
2: triethyl phosphite / 1,2-dichloro-benzene / 24 h / 150 °C / Inert atmosphere
With bis-triphenylphosphine-palladium(II) chloride; sodium carbonate; triethyl phosphite; In ethanol; 1,2-dichloro-benzene; toluene;
|
|
|
Multi-step reaction with 2 steps
1: tetrakis(triphenylphosphine) palladium(0) / tetrahydrofuran; water / 24 h / 80 °C / Inert atmosphere
2: triethyl phosphite / 24 h / 150 °C
With tetrakis(triphenylphosphine) palladium(0); triethyl phosphite; In tetrahydrofuran; water;
|
|
|
Multi-step reaction with 2 steps
1: potassium carbonate; tetrakis(triphenylphosphine) palladium(0) / toluene; ethanol; water / 3 h / 100 °C
2: triphenylphosphine / 1,2-dichloro-benzene / 16 h / 180 °C
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; triphenylphosphine; In ethanol; water; 1,2-dichloro-benzene; toluene;
|
1-Naphthylboronic acid
1-(4-bromo-2-nitrophenyl)naphthalene
1,4-dibromo-2-nitrobenzene
4-bromo-1-iodo-2-nitrobenzene
9-bromo-7-phenyl-7H-benzo[c]carbazole
C28H18BrN
C28H20BNO2
C43H28N4
CAS:15155-41-6
Molecular Formula:C6H2Br2N2S
Molecular Weight:293.97
CAS:419536-33-7
Molecular Formula:C18H14BNO2
Molecular Weight:287.1
CAS:855738-89-5