Your Location:Home >Products >OLED intermediates >Boric acids >855738-89-5
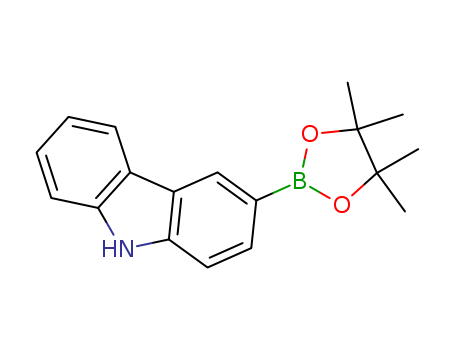

Product Details
Chemical Properties
off-white powder
This article reports a pair of rigid iodide ion macrocyclic receptors ofsyn/anticonfigurations. Single crystal X-ray analysis confirms the structure of thesyn-isomers, and1H Nuclear Magnetic Resonance (1H NMR) titration and ultraviolet-visible (UV-Vis) absorption are used to study the binding affinity of the isomers to halogen ions. Both the isomers show colorimetric and fluorescence recognition towards iodide anions in dichloromethane solution. By solving the association constants, it can be concluded that the interaction between theanti-isomer and iodide ions is relatively weaker than that in thesyn-isomer, and thesyn-isomer has much better recognition selectivity for I?in CH2Cl2than theanti-isomer. The results of the theoretical calculations show thatsyn/anticonfigurations bring about different orientations of the alkyl chains, leading to the larger steric hindrance of theanti-isomer to hinder the combination with iodide ions, and thesynconfigurations with relatively smaller steric hindrance can enhance the binding between the isomers and the iodide anions. A shape persistent macrocycle with a bound conformation can be looked at as a special case of conformational selection, and the results of this study indicate a local induced fit mechanism following a conformational selection in the recognition of iodide anions, and we anticipate that this work will help to understand these two dominant binding mechanisms in molecular recognition and have a certain reference value for designing better anion receptors.
The present invention relates to a triphenylene compound and an organic light emitting device including the same.
An unprecedented and general titanium-catalyzed boration of alkyl (pseudo)halides (alkyl-X, X=I, Br, Cl, OMs) with borane (HBpin, HBcat) is reported. The use of titanium catalyst can successfully suppress the undesired hydrodehalogenation products that prevail using other transition-metal catalysts. A series of synthetically useful alkyl boronate esters are readily obtained from various (primary, secondary, and tertiary) alkyl electrophiles, including unactivated alkyl chlorides, with tolerance of other reducing functional groups such as ester, alkene, and carbamate. Preliminary studies on the mechanism revealed a possible radical reaction pathway. Further extension of our strategy to aryl bromides is also demonstrated.
The present invention relates to an organic luminescent compound represented by chemical formula 1 and an organic electroluminescent device including the same. The organic luminescent compound according to the present invention has excellent luminous efficiency and lifetime properties of material, and thus, enables the manufacturing of an organic electroluminescent device having excellent luminous efficiency while having power efficiency and long lifetime properties. [Chemical formula 1].
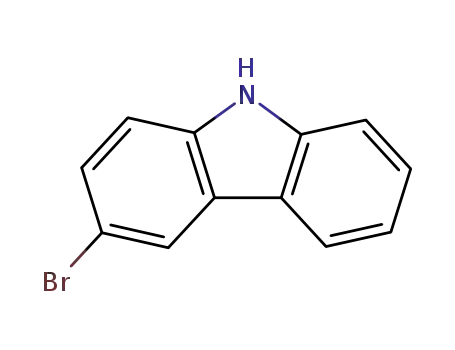
3-bromo-9H-carbazole

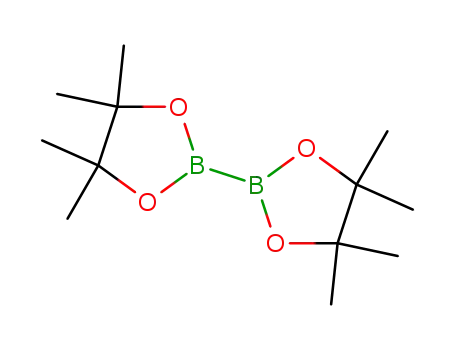
bis(pinacol)diborane

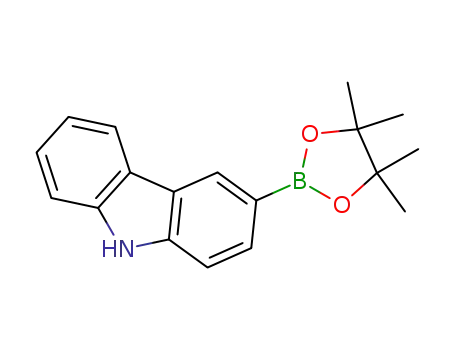
3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)- 9H-carbazole
| Conditions | Yield |
|---|---|
|
With
dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate;
In
1,4-dioxane;
at 80 ℃;
for 24h;
Time;
Inert atmosphere;
|
99% |
|
With
C54H44NO2PPdS; potassium acetate;
In
2-methyltetrahydrofuran;
at 90 ℃;
for 24h;
Inert atmosphere;
Sealed tube;
|
97% |
|
With
potassium acetate;
In
1,4-dioxane;
at 90 ℃;
for 8h;
Inert atmosphere;
|
88% |
|
With
(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate;
In
toluene;
for 5h;
Reflux;
|
85% |
|
With
1,1'-bis-(diphenylphosphino)ferrocene; potassium acetate; palladium diacetate;
In
1,4-dioxane;
at 110 ℃;
for 15h;
Sealed tube;
|
80% |
|
With
(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate;
In
toluene;
for 12h;
Inert atmosphere;
Reflux;
|
70.1% |
|
With
bis-triphenylphosphine-palladium(II) chloride; potassium acetate;
In
1,4-dioxane;
for 2h;
Reflux;
Inert atmosphere;
|
67% |
|
With
1,1'-bis-(diphenylphosphino)ferrocene; potassium acetate; palladium diacetate;
In
dimethyl sulfoxide;
at 90 ℃;
for 24h;
|
64% |
|
With
bis-triphenylphosphine-palladium(II) chloride; potassium acetate;
In
neat (no solvent);
at 110 ℃;
for 24h;
|
52% |
|
With
dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; acetic acid methyl ester;
In
1,4-dioxane;
at 80 ℃;
for 48h;
Sealed tube;
Inert atmosphere;
Darkness;
|
|
|
With
(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate;
In
1,4-dioxane;
for 24h;
Reflux;
|

3-bromo-9H-carbazole

![4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane](/upload/2023/2/d611c0f4-e1f6-4032-a975-703ccb96f00b.png)
4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane


3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)- 9H-carbazole
| Conditions | Yield |
|---|---|
|
4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane;
With
bis(cyclopentadienyl)titanium dichloride; lithium methanolate;
In
neat (no solvent);
for 0.5h;
Inert atmosphere;
Glovebox;
3-bromo-9H-carbazole;
In
neat (no solvent);
at 100 ℃;
for 24h;
Sealed tube;
|
50% |

3-bromo-9H-carbazole

bis(pinacol)diborane
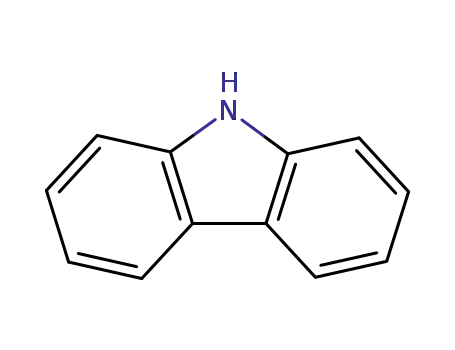
9H-carbazole
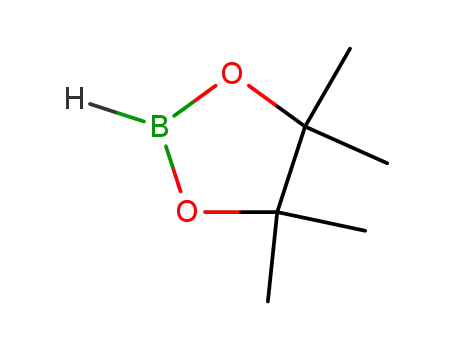
4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane
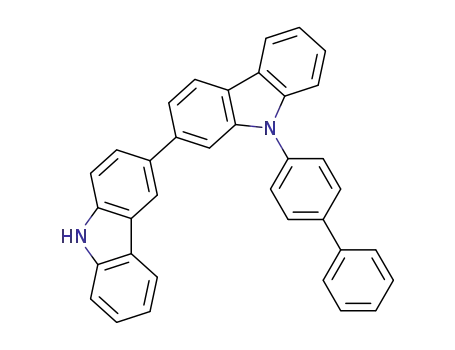
C36H24N2
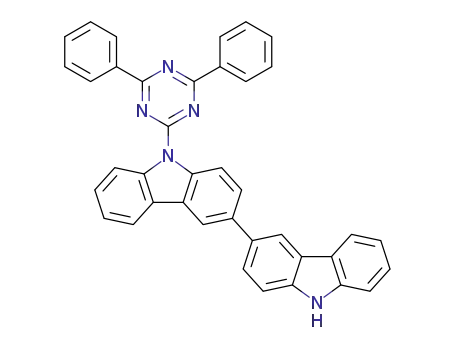
C39H25N5
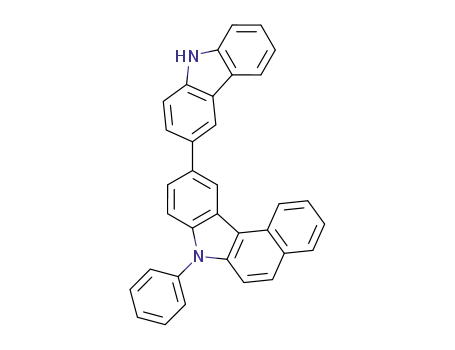
10-(9H-carbazol-3-yl)-7-phenyl-7H-benzo[c]carbazole
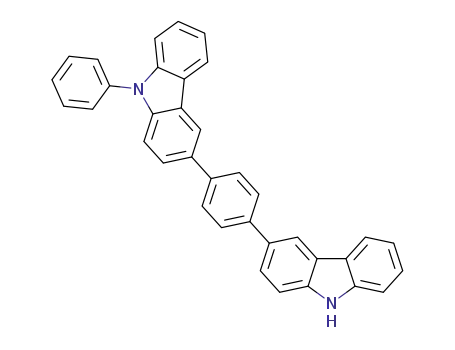
C36H24N2
CAS:63503-60-6
CAS:894791-46-9
Molecular Formula:C24H16BrN
Molecular Weight:398.3