Your Location:Home >Products >Organic phosphines >Tert-butyl phosphines >819-19-2
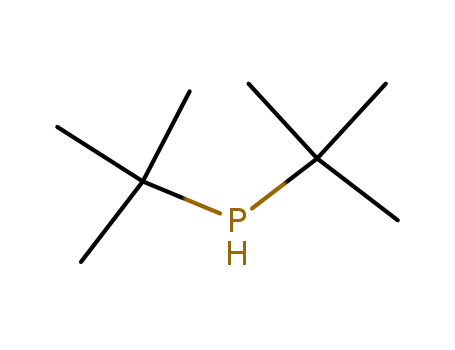

Product Details
Chemical Properties
colourless liquid
Uses
suzuki reaction
InChI:InChI=1/C8H19P/c1-7(2,3)9-8(4,5)6/h9H,1-6H3
While attempts to incorporate secondary phosphine ligands into the first-generation Grubbs catalyst RuCl2(PR3) 2(=CHPh) (GI, R = Cy) were frustrated by decomposition (for HPCy 2) or bulk (for HPtBusu
The reactivity of an anionic phosphanylphosphinidene complex of tungsten(VI), [(2,6-i-Pr2C6H3N)2(Cl)W(η2-t-Bu2P=P)]Li·3DME toward PMe3, halogenophosphines, and iodine was investigated. Reaction of the starting complex with Me3P led to formation of a new neutral phosphanylphosphinidene complex, [(2,6-i-Pr2C6H3N)2(Me3P)W(η2-t-Bu2P=P)]. Reactions with halogenophosphines yielded new catena-phosphorus complexes. From reaction with Ph2PCl and Ph2PBr, a complex with an anionic triphosphorus ligand t-Bu2P-P(-)-PPh2 was isolated. The main product of reaction with PhPCl2 was a tungsten(VI) complex with a pentaphosphorus ligand, t-Bu2P-P(-)-P(Ph)-P(-)-P-t-Bu2. Iodine reacted with the starting complex as an electrophile under splitting of the P-P bond in the t-Bu2P=P unit to yield [(1,2-η-t-Bu2P-P-P-t-Bu2)W(2,6-i-Pr2C6H3N)2Cl], t-Bu2PI, and phosphorus polymers. The molecular structures of the isolated products in the solid state and in solution were established by single crystal X-ray diffraction and NMR spectroscopy. (Chemical Equation Presented).
The reaction of R2P-P(SiMe3)Li (R = tBu, iPr) with the diimido molybdenum complex [(ArN)2MoCl2·dme] (Ar = 2,6-iPr2C6H3; dme = 1,2-dimethoxyethane) yielded the side-on-coordinated
The reaction of R2P-P(SiMe3)Li (R = tBu, iPr) with the diimido molybdenum complex [(ArN)2MoCl2·dme] (Ar = 2,6-iPr2C6H3; dme = 1,2-dimethoxyethane) yielded the side-on-coordinated
[Li+*3DME][Cl((2,6-iPr2C6H3)N)2W(η2-t-Bu2PP)-]

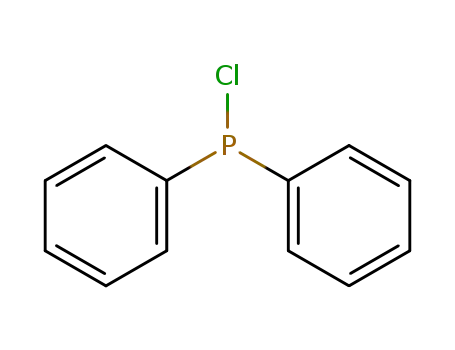
chloro-diphenylphosphine

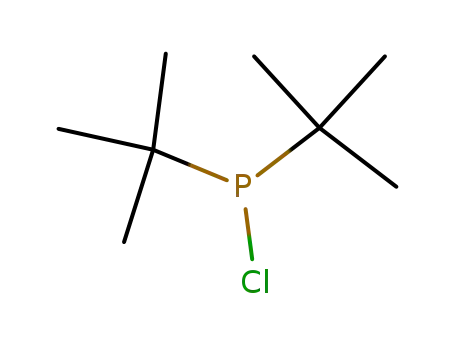
di(tert-butyl)chlorophosphine

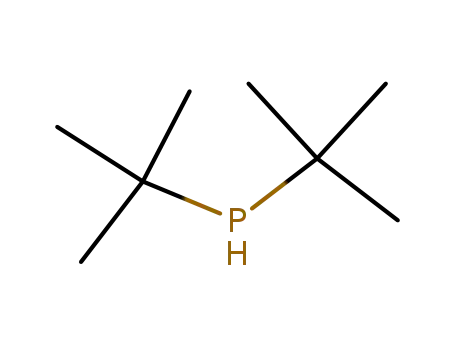
di-tert-butylphosphine

(1,2-η-t-Bu2PPP-t-Bu2)W(2,6-i-Pr2C6H3N)2Cl

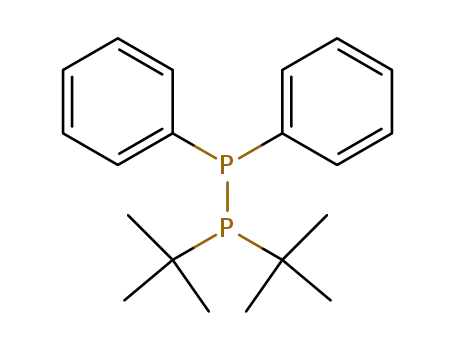
1.1-Diisobutyl-2.2-diphenyldiphosphin

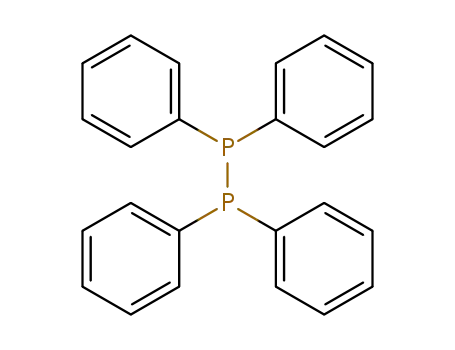
Tetraphenyldiphosphin
| Conditions | Yield |
|---|---|
|
In
1,2-dimethoxyethane;
at -40 - 20 ℃;
for 24h;
Schlenk technique;
|
41 %Spectr. 17 %Spectr. 9 %Spectr. 8 %Spectr. |
[Li+*3DME][Cl((2,6-iPr2C6H3)N)2W(η2-t-Bu2PP)-]

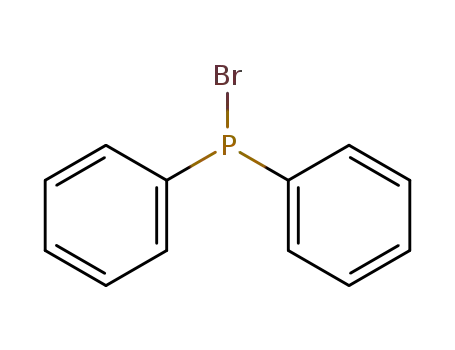
Bromodiphenylphosphin


di-tert-butylphosphine

(1,2-η-t-Bu2PPP-t-Bu2)W(2,6-i-Pr2C6H3N)2Cl

(1,2-η-t-Bu2PPP-t-Bu2)W(2,6-i-Pr2C6H3N)2Br


Tetraphenyldiphosphin
| Conditions | Yield |
|---|---|
|
In
toluene;
at -40 - 20 ℃;
for 24h;
Schlenk technique;
|
54% 19 %Spectr. 19 %Spectr. 14 %Spectr. |
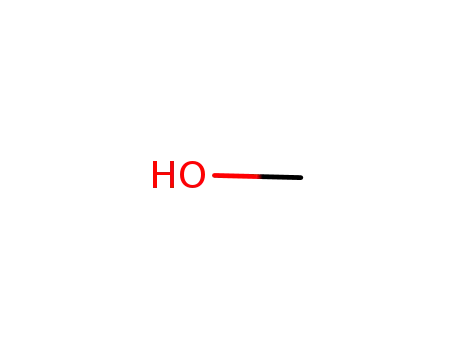
methanol
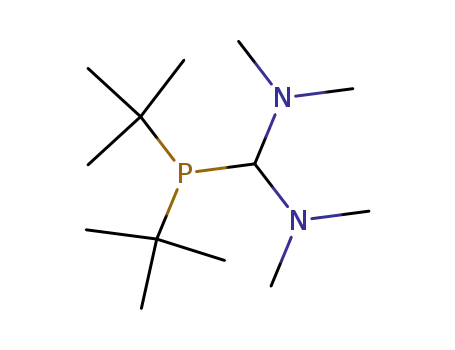
Di-tert-butyl
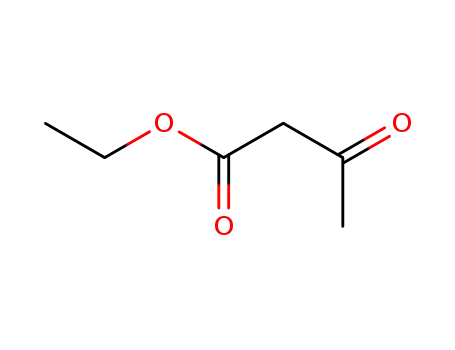
ethyl acetoacetate
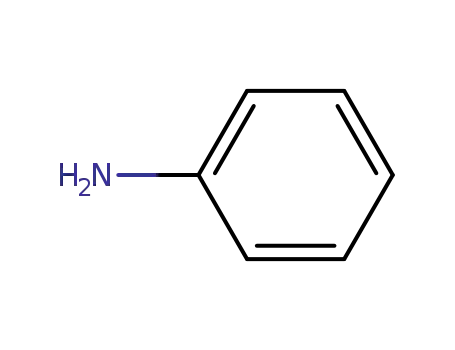
aniline
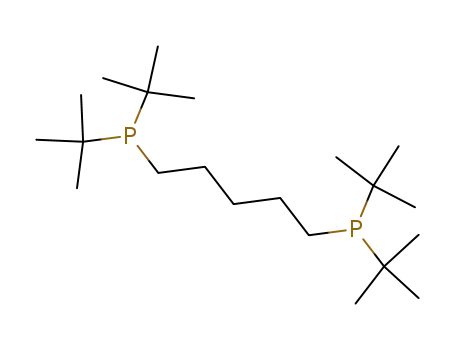
1,5-bis(di-tert-butylphosphino)pentane
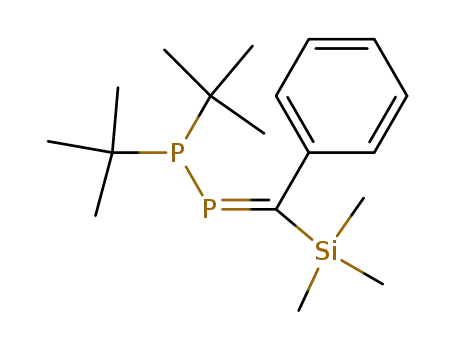
1,1-Di-tert-butyl-2-

di(tert-butyl)chlorophosphine
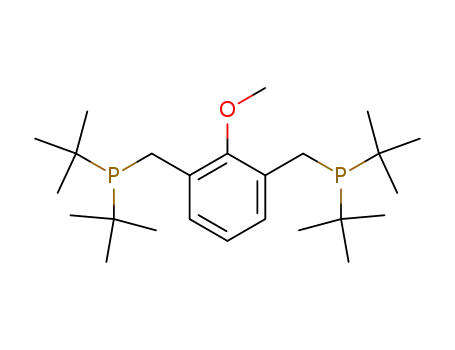
1,3-Bis-[(di-tert-butyl-phosphanyl)-methyl]-2-methoxy-benzene
CAS:564483-19-8
Molecular Formula:C29H45P
Molecular Weight:424.6