Your Location:Home >Products >Organic phosphines >Tert-butyl phosphines >564483-19-8

Product Details
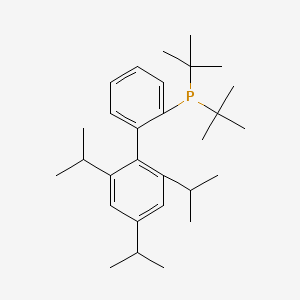
| Description | tBuXPhos [2-Di-tert-butylphosphino-2′,4′,6′-triisopropylbiphenyl] is an air-stable, electron-rich biaryl phosphine ligand developed by the Buchwald group to enhance the reactivity of palladium catalysis during cross-coupling reactions. |
|
Reactions |
Effective ligand for the Pd-catalyzed arylation of pyrazoles, indazoles and amino heterocycles. Ligand used in the Pd-catalyzed synthesis of phenols from aryl halides and KOH. Ligand used in the Pd-catalyzed of benzoic acids from aryl halides and CO2. Ligand used in the Pd-catalyzed trifluoromethylation of vinyl sulfonates. Ligand used in the Pd-catalyzed arylation of nitroacetates. Ligand used in the Pd-catalyzed Suzuki?Miyaura cross-coupling of allylboronates and aryl halides. Ligand used in the Pd-catalyzed cyanation of (hetero)arylchlorides and bromides. Ligand used in the Pd-catalyzed C–N cross coupling of sulfinamides and aryl halides. Ligand used in the Pd-catalyzed arylation of cyanamides. |
|
Chemical Properties |
White to pale yellow |
|
Uses |
2-Di-t-butylphosphino-2',4',6'-tri-i-propyl-1,1'-biphenyl has been used in the preparation of [tBuXPhosAu(MeCN)]BAr4F, a gold catalyst for the intermolecular [2+2] cycloaddition of terminal arylalkynes with substituted alkenes to form functionalized cyclobutenes with high regioselectivity. tBuXPhos is a ligand for Pd-catalyzed C-O and C-N bond formation. It can be used in the following reactions: Palladium-catalyzed Tsuji-Trost substitution and cross-coupling of benzylic fluorides. Palladium-catalyzed C-N cross-coupling of sulfinamides and aryl halides. Palladium-catalyzed rapid methoxylation and deuteriomethoxylation of bromo-chalcones. |
The invention discloses a method for syn...
A mixture of tris(dibenzylideneacetone)dipalladium (29.9 kg, 3.26 mol) and 2-di-t-butylphosphino-2′,4′,6′-tri-i-propyl-1,1′-biphenyl (7.80 kg, 18.4 mol) was added to the reaction …
The first general method for the Pd-cata...
di(tert-butyl)chlorophosphine

2-bromo-1-chlorobenzene

2,4,6-triisopropyl-1-bromobenzene

tert-butyl XPhos
| Conditions | Yield |
|---|---|
|
2-bromo-1-chlorobenzene; 2,4,6-triisopropyl-1-bromobenzene; With magnesium; In tetrahydrofuran; for 2h; Inert atmosphere; Reflux;
di(tert-butyl)chlorophosphine; With tetrakis(triphenylphosphine) palladium(0); In tetrahydrofuran; at 20 ℃; for 5.5h; Reagent/catalyst; Reflux; Inert atmosphere;
|
94% |
|
2,4,6-triisopropyl-1-bromobenzene; With magnesium; ethylene dibromide; In tetrahydrofuran; at 65 ℃; for 1h;
2-bromo-1-chlorobenzene; In tetrahydrofuran; at 65 ℃; for 1h;
di(tert-butyl)chlorophosphine; With copper(l) chloride; In tetrahydrofuran; at 20 ℃; for 20h;
|
32% |
di-tert-butylphosphine

2,4,6-triisopropylphenylboronic acid

2,3-dibromobenzene

tert-butyl XPhos
| Conditions | Yield |
|---|---|
|
di-tert-butylphosphine; 2,3-dibromobenzene; With bis[di-tert-butyl(4-dimethylaminophenyl)phosphine]palladium(0); sodium carbonate; In toluene; at 80 ℃; for 8h; Inert atmosphere;
2,4,6-triisopropylphenylboronic acid; In toluene; at 100 ℃; for 12h; Inert atmosphere;
|
86% |
di(tert-butyl)chlorophosphine
2-bromo-1-chlorobenzene
2,4,6-triisopropyl-1-bromobenzene
di-tert-butylphosphine
tert-butyl 2-(benzamido)-4-(3-methoxyphenoxy)benzoate
tert-butyl 2-(benzamido)-4-(3-nitrophenoxy)benzoate
tert-butyl 2-(benzamido)-4-(3,5-difluorophenoxy)benzoate
tert-butyl 2-(benzamido)-4-(4-methylphenoxy)benzoate
CAS:98327-87-8
Molecular Formula:C<sub>44</sub>H<sub>32</sub>P<sub>2</sub>
Molecular Weight:622.7
CAS:84680-95-5
CAS:819-19-2