Your Location:Home >Products >Organic phosphines >Other organic phosphines >1038-95-5
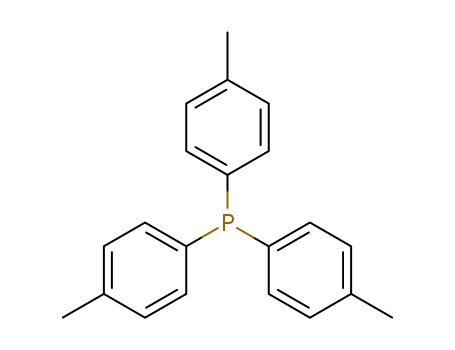

Product Details
Chemical Properties
white to light yellow crystal powde
Uses
suzuki reaction
Uses
Tri(p-tolyl)phosphine is used as pharmaceutical intermediate.
InChI:InChI=1/C21H21P/c1-16-4-10-19(11-5-16)22(20-12-6-17(2)7-13-20)21-14-8-18(3)9-15-21/h4-15H,1-3H3
The basicities of the triarylphosphines P(4-XC6H4)3 (X = Cl, F, H, CH3, CH3O, (CH3)2N), P(3-CH3C6H4)3, and P(2-CH3C6H7)3 as well as the trialkylphosphines P(t-Bu)3 and PCy3 have been measured by the nitromethane titration method.The range of basicity available by aryl substitution is very large, being pKa = 8. 65 for X = (CH3)2N to 1.03 for X = Cl.The most basic phosphine is P(t-Bu)3 whose pKa = 11.40.The measured basicities correlate well wit ?p, ?Φ, and ν as well as with the lone pair ionisation potentials of the triarylphosphines.Generally the 1H,31P, and 13C nmr spectral parameters of the free and protonated phosphines do not correlate well with pKa.
The metal-free reduction of phosphane oxides with molecular hydrogen (H2) using oxalyl chloride as activating agent was achieved. Quantum-mechanical investigations support the heterolytic splitting of H2 by the in situ formed electrophilic phosphonium cation (EPC) and phosphane oxide and subsequent barrierless conversion to the phosphane and HCl. The reaction can also be catalyzed by the frustrated Lewis pair (FLP) consisting of B(2,6-F2C6H3)3 and 2,6-lutidine or phosphane oxide as Lewis base. This novel reduction was demonstrated for triaryl and diaryl phosphane oxides providing access to phosphanes in good to excellent yields (51–93 %).
-
The diastereoselective synthesis of anti-homoallylic alcohols bearing conjugated (Z)-enynes through a palladium-catalyzed three-component reaction is described. This reaction features a broad substrate scope, good functional group compatibility, and high levels of (Z)-alkene stereocontrol. In this reaction, Pd(0) functions as a catalyst in two fundamental steps of the tandem sequence: 1) the generation of a borylated π-allylpalladium species from bifunctional conjunctive reagents, inducing umpolung allylation of aldehydes, and 2) C(sp2)?C(sp) cross-coupling. Further transformations of the obtained products highlight their synthetic utility. (Figure presented.).
Radiolabelled lipophilic cations that accumulate in mitochondria according to the magnitude of the mitochondrial membrane potential can be used to report non-invasively on mitochondrial dysfunction in cardiovascular disease, cardiotoxicity, and cancer. While several such cations are already commercially available for SPECT imaging, PET offers greater promise in terms of sensitivity, resolution, and capacity for dynamic imaging and pharmacokinetic modelling. We have therefore synthesised a series of three triarylphosphonium-functionalised DO3A chelators for positron emitter gallium-68, with differing alkyl-functionalisation motifs to provide opportunities for tunable lipophilicity as a means of optimising their pharmacokinetics. To assess their capacity to report on mitochondrial membrane potential, we assessed their pharmacokinetic profiles in isolated tumour cells and isolated perfused rat hearts before and after mitochondrial depolarisation with the ionophore CCCP. All three compounds radiolabelled with over 97% RCY and exhibited log?D values of between ?3.12 and ?1.81. In vitro assessment of the uptake of the radiotracers in cultured tumour cells showed a three-fold increase in uptake compared to unchelated [68Ga]Ga(iii). However, each complex exhibited less than 1% retention in healthy hearts, which was not significantly diminished by mitochondrial depolarisation with CCCP. This preliminary work suggests that while this approach is promising, the lipophilicity of this class of tracers must be increased in order for them to be useful as cardiac or cancer imaging agents.
Direct reductions of phosphine oxides to the corresponding phosphines were performed successfully by using Mg/Me3SiCl/DMI system. The reduction proceeded under mild conditions and was applicable to a wide range of phosphine oxides; triarylphosphine oxides, alkyldiarylphosphine oxides, and dialkylarylphosphine oxides gave the corresponding phosphines in good to excellent yields.
-
The (COCl)2/Hantzsch ester is found to be an effective system for the metal-free reduction of tertiary phosphine oxides. The reaction proceeds under mild conditions, and is applicable to triarylphosphine oxides and alkyldiarylphosphine oxides to produce the corresponding tertiary phosphines in good to excellent yields. This new finding provides a practical, convenient and metal-free method for the reduction of tertiary phosphine oxides to tertiary phosphines, and shows potential application in organic synthesis.
Electroreduction of triphenylphosphine oxide to triphenylphosphine in an acetonitrile solution of tetrabutylammonium bromide in the presence of chlorotrimethylsilane was performed successfully in an undivided cell fitted with a zinc anode and a platinum cathode under constant current. A plausible mechanism involving, (1) one-electron reduction of triphenylphosphine oxide generating the corresponding anion radical [Ph3P-O-], (2) subsequent reaction with chlorotrimethylsilane affording the (trimethylsiloxy)triphenylphosphorus radical [Ph3P-OSiMe 3], and (3) further one-electron reduction followed by P-O bond fission leading to triphenylphosphine is proposed. In a similar manner, electroreduction of some triarylphosphine oxides and alkyldiarylphosphine oxides was executed to give the corresponding phosphine derivatives in good to moderate yields. Georg Thieme Verlag Stuttgart · New York.
A new method for the iodine-catalyzed reduction of phosphine oxides with phosphites at room temperature is reported. The mild reaction conditions, scalability, and simple purification requirements render it a method of choice for the large-scale production and facile regeneration of a variety of phosphines. Mechanistic studies, supported by DFT calculations of the oxygen transfer between the starting phosphine oxide and the phosphite reagent, are also presented. Such transmutations of phosphorus species were previously unknown.
Palladium (II) catalyzed cleavage of phenyl-antimony and phenyl-phosphorus groups of Ph3Sb and Ph3P under carbon-dioxide or CO/NO atmosphere leading to benzoic acid, has been demonstrated.
Aryl halides, ArX (Ar = Ph, 2-, 3- and 4-Tol, 1- and 2-Np, 4-C6H4CONH2; X = F, Cl, Br), rapidly and exothermically (100–180 °C, 0.5–2 h) react with red phosphorus in superbase systems KOH/L, where L is a polar nonhydroxylic complexing solvent (ligand), such as NMP, DMSO, HMPA, to afford the corresponding triarylphosphines (Ar3P) in up to 74 % yield (for X = F). Thus, three consecutive reactions of SNAr (aromatic nucleophilic substitution) to form the three C(sp2)–P bonds are realized in one pot. The synthesis is mostly chemoselective (with rare exception): neither mono- nor diphosphines have been isolated. The best results were attained when aryl fluorides were treated with red phosphorus (Pn) in the KOH/NMP superbase system. This environmentally friendly, PCl3-free synthesis of Ar3P from available starting materials opens an easy and straightforward access to triarylphosphines, which are important ligands, synthetic auxiliaries, and components of high-tech- and medicinally oriented complexes.
Equilibrium constants (K) for reactions between acids and the conjugate base forms of a number of phosphonium salts, [HPR3][BF4], and iron hydrides, [Fe(CO)3H(PR3)2][BF 4], in CD2Cl2 have been determined by means of 31P and 1H NMR spectroscopy at 20°C. The anchor compound chosen for pKCD2Cl2 determinations was [HPCy 3][BF4] with a pKCD2Cl2 value of 9.7, as assigned by literature convention (Cy: cyclohexyl). A continuous scale of pKCD2Cl2 values covering the range from 9.7 to -3 was created and correlated with the ΔH values reported by Angelici and co-workers and literature pKa values. The pKCD2Cl2 values for 15 other hydride or dihydrogen complexes of the iron group elements and of diethyl ether were also placed on this scale. The crystal structures of [Fe(CO) 3H(PCy2Ph)2][BF4] and [Fe(CO) 3(PCy2Ph)2] revealed that the frans-oriented, bulky, unsymmetrical phosphane ligands distort the equatorial plane of the complexes. The acidity of iron carbonyl hydrides is an important feature of the reactions of iron hydrogenase enzymes.
Chlorobenzenes are important starting materials for the preparation of commercially valuable triarylphosphines and tetraarylphosphonium salts, but their use for the direct arylation of elemental phosphorus has been elusive. Here we describe a simple photochemical route toward such products. UV-LED irradiation (365 nm) of chlorobenzenes, white phosphorus (P4) and the organic superphotoreductant tetrakis(dimethylamino)ethylene (TDAE) affords the desired arylphosphorus compounds in a single reaction step.
The invention aims to provide an aryl phosphine oxide compound as a raw material, wherein P=O keys are activated by an acid anhydride and alkali is continued. The preparation of the phosphine (III) compound is carried out under the action of a crown ether and a reducing agent. The method has the advantages of cheap and easily available raw materials, simple operation, high atomic economy and the like. Compared with a traditional reduction mode, the method is ingenious in design, waste emission is reduced, separation of intermediate products is omitted, and related reagents such as silicon hydrogen, aluminum, boron and the like with higher price can be avoided. And the reaction suitability is extensive.
Palladium-catalyzed C-P bond formation reaction of ArBr/ArOTf using acylphosphines as differential phosphination reagents is reported. The acylphosphines show practicable reactivity with ArBr and ArOTf as the phosphination reagents, though they are inert to the air and moisture. The reaction affords trivalent phosphines directly in good yields with a broad substrate scope and functional group tolerance. This reaction discloses the acylphosphines' capability as new phosphorus sources for the direct synthesis of trivalent phosphines.
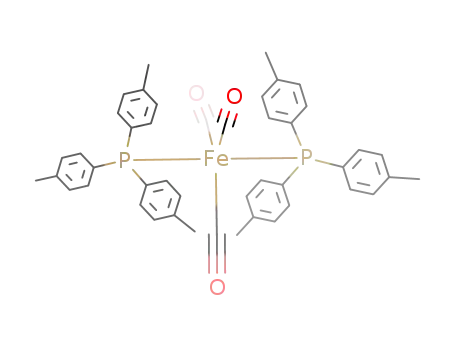
tricarbonyl bis(tri-p-tolylphosphine) iron

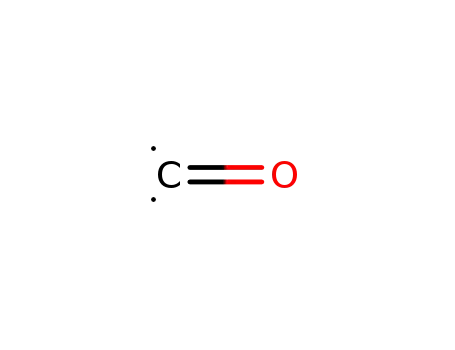
carbon monoxide

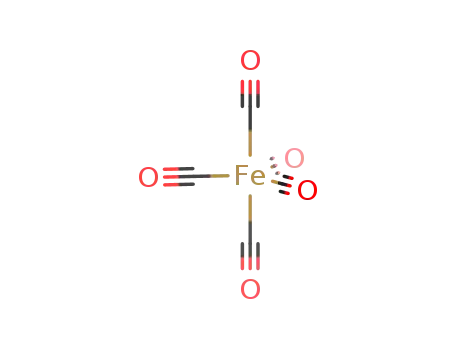
iron pentacarbonyl

(CO)4FeP(C6H4CH3-p)3

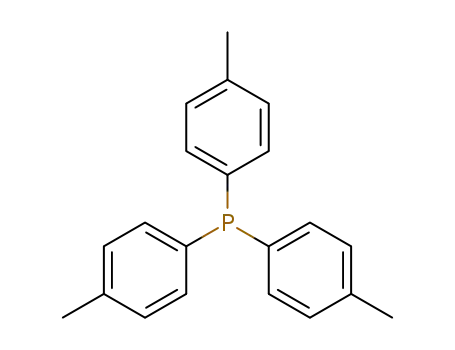
Tri(p-tolyl)phosphine
| Conditions | Yield |
|---|---|
|
In
further solvent(s);
Irradiation (UV/VIS); under CO in hydrocarbon;
|
0% |
|
|
phenacyl-tri-p-tolyl-phosphonium; tri-p-tolyl-phenacyl-phosphonium chloride

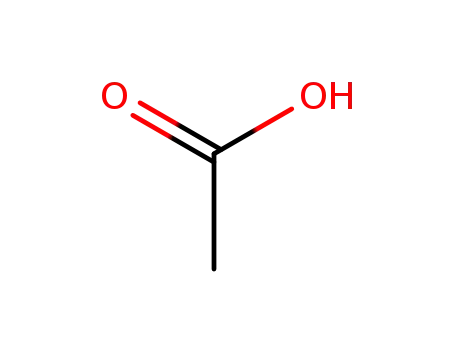
acetic acid

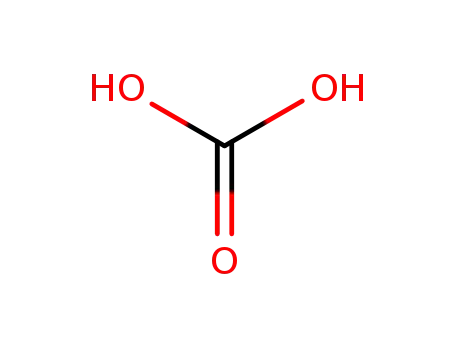
carbonic-acid


Tri(p-tolyl)phosphine
| Conditions | Yield |
|---|---|
|
|
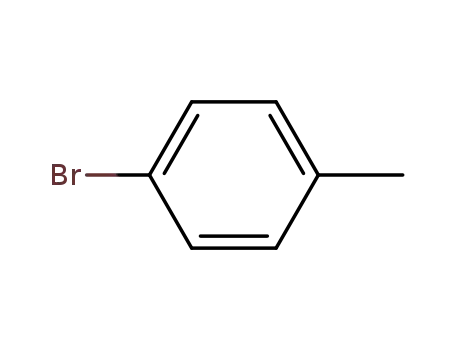
para-bromotoluene
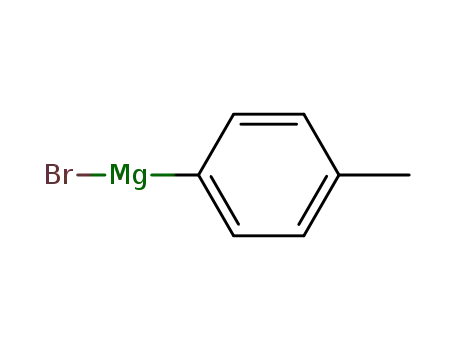
para-methylphenylmagnesium bromide
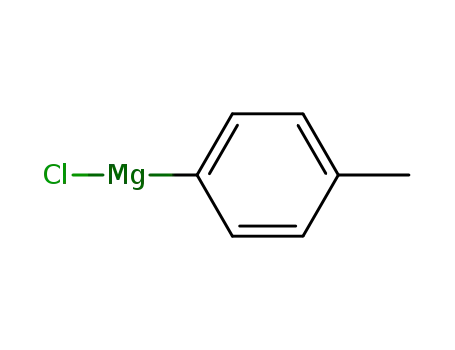
4-tolylmagnesium chloride

p-tolyllithium
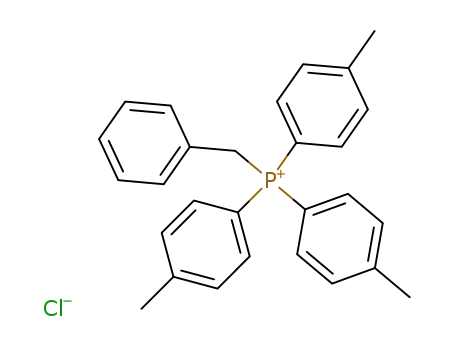
benzyl-tri-p-tolyl-phosphonium; chloride
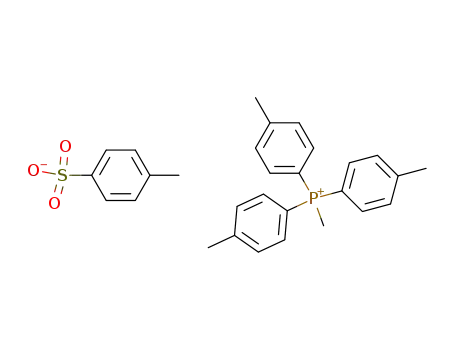
methyltris(4-methylphenyl)phosphonium tosylate
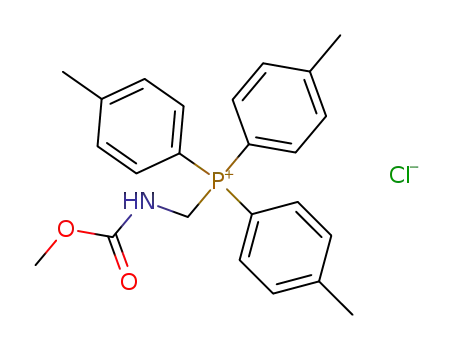
(Methoxycarbonylamino-methyl)-tri-p-tolyl-phosphonium; chloride
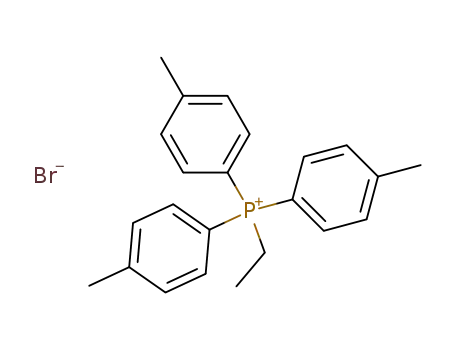
tri-p-tolylethylphosphonium bromide
CAS:739-58-2
CAS:240417-00-9
CAS:76189-55-4
Molecular Formula:C44H32P2
Molecular Weight:622.7