Your Location:Home >Products >OLED intermediates >Thiophenes >668983-97-9

Product Details
|
Chemical Properties |
off-white powder |
| Description | Dibenzothiophene-2-boronic Acid is an organometallic compound consists of a dibenzothiophene core with a boronic acid functional group attached to the 2-position. Dibenzothiophene-2-boronic Acid is a solid at room temperature and is commonly used as a precursor or building block in the synthesis of various organic and organometallic materials. Dibenzothiophene-2-boronic Acid is of interest due to its potential applications in areas such as organic electronics, catalysis, and material science, where the boron-containing functional group can participate in various chemical reactions and interactions. |
|
Uses |
Dibenzothiophene-2-boronic acid is a useful reagent for organic synthesis and other chemical processes. |
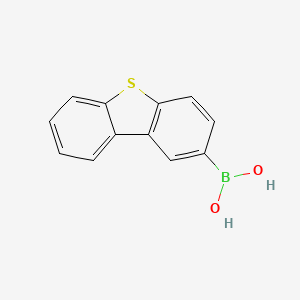
Isomeric SMILES: B(C1=CC2=C(C=C1)SC3=CC=CC=C32)(O)O
InChIKey: CSLSCVHILGCSTE-UHFFFAOYSA-N
InChI: InChI=1S/C12H9BO2S/c14-13(15)8-5-6-12-10(7-8)9-3-1-2-4-11(9)16-12/h1-7,14-15H
We report herein versatile, transition m...
The triad types of molecules with variou...
… hydrochloric acid to obtain dibenzothiophene-2-boronic acid. … acid (35 %) gave a white precipitate, which was collected by centrifugation. The crude dibenzothiophene-2-boronic acid …
PROBLEM TO BE SOLVED: To provide a triaz...
2-bromodibenzothiophene

dibenzo[b,d]thien-2-ylboronic acid
| Conditions | Yield |
|---|---|
|
2-bromodibenzothiophene; With n-butyllithium; In tetrahydrofuran; hexane; toluene; at -70 ℃; for 1h; Inert atmosphere;
With Triisopropyl borate; In tetrahydrofuran; hexane; toluene; at 20 ℃; for 6h;
With hydrogenchloride; In tetrahydrofuran; hexane; water; toluene; for 0.5h;
|
95% |
|
2-bromodibenzothiophene; With n-butyllithium; In tetrahydrofuran; hexane; at -78 ℃; for 1h; Inert atmosphere;
With Triisopropyl borate; In tetrahydrofuran; hexane; at -78 - 20 ℃; for 12h;
|
92% |
|
With n-butyllithium; Trimethyl borate; sulfuric acid; water; In hexane; at -78 - 20 ℃;
|
88% |
|
With n-butyllithium; diethyl ether; Behandeln des Reaktionsprodukts mit Triisopropylborat in Aether bei -60grad und Behandeln des Reaktionsgemisches mit Wasser;
|
2-bromodibenzothiophene

Trimethyl borate

dibenzo[b,d]thien-2-ylboronic acid
| Conditions | Yield |
|---|---|
|
2-bromodibenzothiophene; With n-butyllithium; In tetrahydrofuran; hexane; at -78 - 0 ℃; for 1h;
Trimethyl borate; In tetrahydrofuran; hexane; at -78 - 20 ℃; for 12h;
|
82% |
|
2-bromodibenzothiophene; With n-butyllithium; In tetrahydrofuran; at -78 ℃; for 1h; Inert atmosphere;
Trimethyl borate; In tetrahydrofuran; at 20 ℃; for 3h; Inert atmosphere;
With hydrogenchloride; In tetrahydrofuran; water; for 0.5h; Inert atmosphere;
|
80% |
|
2-bromodibenzothiophene; With n-butyllithium; In tetrahydrofuran; hexane; at -78 - 0 ℃; for 1h;
Trimethyl borate; In tetrahydrofuran; hexane; at -78 - 20 ℃; for 12h;
|
|
|
2-bromodibenzothiophene; With n-butyllithium;
Trimethyl borate;
With hydrogenchloride;
|
2-bromodibenzothiophene
dibenzothiophene
Triisopropyl borate
triisopropylborane
C44H27N3S
CAS:955959-91-8