Your Location:Home >Products >OLED intermediates >Fluorenes >196207-58-6
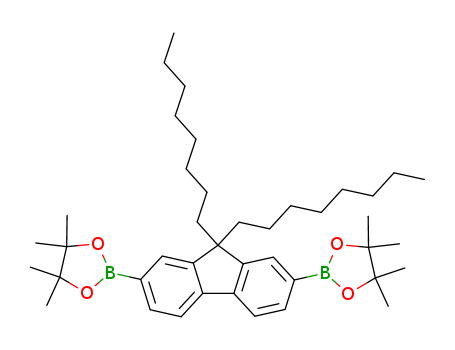

Product Details
Description
2,2'-(9,9-dioctyl-9H-fluorene-2,7-diyl)bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane) is a precursor for the synthesis of polymer semiconductors in the application of OLED, PLED, OFET and Polymer Solar Cells.
Uses
9,9-Di-n-octylfluorene-2,7-diboronic acid bis(pinacol) ester is a reactant in the synthesis of 6,6-(5,5-(9,9-dioctyl-9H-fluorene-2,7-diyl)bis(thiophene-5,2-diyl))bis(2,5-bis(2-ethylhexyl)-3-(thiophen-2-yl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione) (DPP1), a solution-processable nonfullerene electron acceptor used in organic solar cells.
A series of polyfluorene copolymers composed of 9,9-dioctylfluorene (DOF) and 2,3-diphenylfumaronitrile (DPFN), poly(DOF-co-DPFN)s, have been synthesized through a palladium (0)-catalyzed Suzuki coupling reaction, and their light-emitting properties have
Two fluorene-based copolymers (PF-33F and PF-50F) with p-difluorophenylene units in the backbone were synthesized. In comparison with the reference poly(9,9-dioctylfluorene) (PFO), the introduction of p-difluorophenylene units not only increased the fluorescent quantum yields, but also improved the spectra purity and stability of these deep blue emitting copolymers. The famous green emission band at 520 nm from fluorenone defects was never detected for these copolymers even after they were thermal annealed in air at 150 °C. Organic light-emitting diodes were fabricated using them as emitting layer and pure blue electroluminescence was obtained. It was observed that PF-33F based device exhibited much higher current density and brightness than PF-50F and PFO devices. A maximum external quantum efficiency of 1.14% (1.14 cd A-1) and the CIE (0.16, 0.13) were achieved for PF-33F device, which are among the best performance for polyfluorenes reported so far.
Two dithienocyclopentafluorene-based small-molecule acceptors (SMAs) were developed that feature methylene-functionalized conjugated side chains, to study the effect of arylmethylene substitution and its number on structure, optoelectronic properties and device performance. Results showed that two SMAs have better absorption properties and planarity, lower bandgaps and higher LUMOs compared with the control SMA without conjugated side chains. The synthesized SMAs were tested in polymer solar cells for examples of their applicability. This work argues that the introduction of methylene-functionalized conjugated side chains has great potential in tuning molecular structure, optoelectronic properties, device physics and photovoltaic performance of SMAs.
A series of novel blue light-emitting polymers were designed and synthesized by incorporating a blue styrylarylene amine (DV) chromophore into the backbone of poly(9,9-dioctylfluorene). All the resultant polymers exhibit blue emission peaking at around 46
A facile method to prepare hydrophilic polymers by a postpolymerization nucleophillic aromatic substitution reaction of fluoride on an emissive conjugated polymer (CP) backbone is reported. Quantitative functionalization by a series of monofunctionalized ethylene glycol oligomers, from dimer to hexamer, as well as with high molecular weight polyethylene glycol is demonstrated. The length of the ethylene glycol sidechains is shown to have a direct impact on the surface wettability of the polymer, as well as its solubility in polar solvents. However, the energetics and band gap of the CPs remain essentially constant. This method therefore allows an easy way to modulate the wettability and solubility of CP materials for a diverse series of applications.
A new copolymer, constituted by the regular alternation of a fluorene derivative with a 1,1-dioxothiophene moiety, has been synthesised via Suzuki coupling. The molecular, thermal, structural, and photophysical characterisations have been performed. The material showed two dimensional order, enhanced by annealing; molecular modelling calculations afforded a consistent structural model accounting for the optical data. In comparison with both polyfluorenes and 1,1-dioxothiophene oligomers the unusual photoluminescence quantum yields observed in solution and in the solid state can be related to a significant contribution of internal conversion to the deactivation process in solution, and the formation of intra-chain hydrogen-bonds, due to a peculiar chain conformation, in the solid state. LED devices based on this new copolymer showed the highest efficiency compared with similar copolymers containing both fluorene and 1,1-dioxothiophene functionalities.
We have synthesized and characterized a polyfluorene derivative composed of octyl-substituted thieno[3,2,-b]thiophene and 2,1,3-benzothiadiazole as an acceptor unit. The best power conversion efficiencies of the polymer were showed with 1.63% and 2.31% by using PCBM and PC71BM, respectively. Copyright
A novel series of light-emitting copolymers derived from 9,9-dioctylfluorene (DOF) and 2,1,3-benzoselenadiazole (BSeD) is prepared by means of palladium-catalyzed Suzuki coupling reaction. The feed ratios of DOF to BSeD were 50:50, 85:15, 92:8, and 98:2, respectively. All of the copolymers are soluble in common organic solvents and highly fluorescent in solid state. Devices from such copolymers emit orange-red light with λmax = 570-600 nm. The maximal EL emissions of the devices slightly red-shifted gradually with increasing BSeD's contents. The maximal external quantum efficiency of the polymer light-emitting devices (PLED) reaches 1.0%, which indicates that this new seleno-containing EL polymer based on fluorene and benzoselenadiazole is a promising candidate for fabricating PLEDs.
4-Hexylbithienopyridine has been prepared as a novel electron-accepting monomer for conjugated polymers. To test its electronic properties, alternating copolymers with fluorene and indenofluorene polymers have been prepared. The copolymers displayed reduction potentials about 0.5 V lower than for the corresponding fluorene and indenofluorene homopolymers, indicating much improved electron-accepting properties. Analysis of the microscopic morphology of thin films of the copolymers by AFM shows that they lack the extensive supramolecular order seen with the homopolymers, which is attributed to the bithienopyridine units disrupting the π-stacking. LEDs using these polymers as the emitting layer produce blue-green emission with low turn-on voltages with aluminum electrodes confirming their improved electron affinity. The indenofluorene copolymer displayed an irreversible red shift in emission at high voltages, which is attributed to oxidation of the indenofluorene units. This red shift occurred at higher potentials than for indenofluorene homopolymers in LEDs, suggesting that the heterocyclic moieties offer some protection against electrically promoted oxidation.
A variety of novel light-emitting copolymers derived from 9,9-dioctylfluorene (DOF) and 2,1,3-naphthozoselenadiazole (NSeD) were prepared by the palladium-catalyzed Suzuki coupling reaction. The feed ratios of DOF to NSeD were 99.9:0.1, 99.5:0.5, 99:1, 98:2, 95:5, and 85:15. All of the polymers are soluble in common organic solvents and highly fluorescent in solid state. Devices based on the copolymers emit saturated red light, and the emission slightly red-shifted gradually with increasing NSeD's contents. The maximal external quantum efficiency of the polymer light-emitting devices (PLED) reaches 3.1%, and luminous efficiency is greater than 1.0 cd/A with emission maximum at 657 nm and Commission Internationale de L'Eclairage (CIE) coordinates of (0.64, 0.33). This is the highest efficiency with saturated red emission for a single-layer device with nonblend type emitter reported so far in the scientific literature. This indicates that the new EL polymers based on fluorene and naphthoselenadiazole are promising as a red emitter in polymer light-emitting displays.
A new class of chiral atropoisomeric (P, N) ligand precursors has been obtained with excellent diastereoselectivities and high yields through diastereoselective metal-free intramolecular radical oxidative C-H amination with chiral phosphamide as the auxil
A series of novel praseodymium(Pr)-coordinated polymers with fluorene, phthalimide and bipyridine moieties in the main chain were synthesized via a coordination reaction and palladium-catalyzed Suzuki coupling polycondensation. Their structures, optical features and memory performance have been well studied. A resistive switching device with the configuration of ITO/polymer/Al was constructed using a spin-coating process. The device exhibits nonvolatile write-once-read-many-times (WORM) memory behavior. Based on the electrochemical properties and theoretical calculations of the polymers, the effects of the phthalimide moiety and neutral Pr complex on the polymer memory device performance were investigated. Our work offers valuable clues on the development of polymer memory devices.
End-capped effects are important in modifying the structures of donor materials, with the purpose of regulating material properties and achieving high open-circuit voltage (VOC). A series of acceptor-π-donor-π-acceptor (A-π-D-π-A) type small-molecule donor (SMD) materials for bulk-heterojunction (BHJ) organic solar cells with PC71BM as electron acceptor have been designed and synthesized. Three novel small molecules are denoted as flu(3TRD)2, flu(3TCN)2 and flu(3TIN)2, with dioctyl-substituted fluorene as core, 3,3″-dioctyl-2,2':5′,2″-terthiophene as π-bridge, terminated by rhodanine, malononitrile and 1,3-indanedione, respectively. Improvements of photovoltaic performance have been successfully realized via tuning terminal electron-withdrawing units. The power conversion efficiency (PCE) of the devices as-cast prepared from flu(3TRD)2, flu(3TCN)2 and flu(3TIN)2 increases in turn, which is ascribed to incremental optical absorption thereby promoting short-circuit current density (JSC). Particularly, flu(3TCN)2 shows deeper LUMO and HOMO energy levels than flu(3TRD)2 and flu(3TIN)2 due to the strong electron-withdrawing character of malononitrile. As expected, the device as-cast based on flu(3TCN)2 exhibits a reasonable high VOC of 1.07 V. In addition, a satisfactory PCE of 7.06percent has been obtained for flu(3TCN)2 based device with dichloromethane vapor treatment.
To explore the influence of push-pull chromophores on properties of emitter in organic light-emitting devices (OLEDs), an acceptor-donor-acceptor (A-D-A)-based dinuclear iridium (III) complex of (dfppy)4Ir2(dipic-FL) was synthesized via Suzuki coupling reaction, in which dfppy is 2-(2,4-difluorophenyl)pyridine and dipic-FL is 2,7-di(5-pyridyl-2-carboxyl)- 9,9-dioctyl-9H-fluorene. An intense emission peak at about 480 nm resulting from the (dfppy)2Ir(pic) chromophore and a weak long-wavelength emission band at 580-660 nm attributed to intramolecular charge transfer transition were exhibited for (dfppy)4-Ir2(dipic-FL) in dichloromethane solution. But a remarkably hypsochromic photoluminescence profile with an intense characteristical emission peak at 422 nm was observed, which is attributed to the intraligand (IL) π-πexcited states in its thin film. White emission with a maximum luminance of 1040 cd/m2 and current efficiency of 1.2 cd/A was obtained in its single-emissive-layer (SEL) OLEDs with a configuration of ITO/PEDOT:PSS/ (dfppy)4Ir2(dipic-FL) (10 wt%):TCTA/TPBi/LiF/Al. To our knowledge, this is one of the best examples in term of dinuclear iridium complex as single dopant in the high-performance white-emitting SEL-OLEDs.
In this paper, we demonstrated a facile photopatterning method that uses photocrosslinkable polyfluorene to fabricate micro-sized photopatterns on transparent indium tin oxide substrate for neuronal patterning. The modified poly(ethyleneimine) (m-PEI) with trimethoxysilane moiety was chemically attached to the hydroxyl group-terminated ITO surface and then the photopatternable polyfluorene derivative was spin coated as a cell-repellent layer onto the m-PEI-coated surface. The well-defined micropatterns were easily created over an entire surface by photocrosslinking of bromoalkyl-substituted polyfluorene (Br-PF) via the radical coupling reaction of a C-Br bond under UV irradiation without an initiator. UV-Vis absorbance, photoluminescence, ATR-FTIR and X-ray photoelectron spectroscopy were used to confirm the photocrosslinking process and the surface composition before and after the photocrosslinking of polyfluorene. The pairing of adhesive m-PEI and repulsive Br-PF effectively guided the neurite outgrowth and controlled neurite extension from individual neurons to the pre-patterned direction with excellent pattern fidelity. Guided neuronal cells were maintained for at least 25 days in vitro without any detachment of neuronal cells during cell culture. A photopatternable polyfluorene derivative in combination with cell-adhesive m-PEI is proved to be an effective way to modify the electrode surface to achieve single cell level neuronal networks.
Two novel phenanthroimidazole derivatives were synthesized for efficient three-photon excited fluorescence and absorption. By introducing spirobi [9H-fluorene] to replace fluorene, the three-photon-excited fluorescence and cross-section are significantly
A group of novel fluorene-based copolymers were synthesized and characterized. The trifluoromethylphenylene unit was introduced into the copolymer backbone and its content ratio was varied from 10 mol% to 50 mol%. The electronic bandgap of the copolymer increases regularly with increasing trifluoromethylphenylene ratio. In contrast to pure polyfluorene, the deep-blue fluorescence of these copolymers is quite stable and not contaminated by the well-known green emission associated with fluorenone defects. The copolymers were used as an emitting layer to fabricate organic light-emitting diodes with a pure blue electroluminescence with a CIE coordinate y ≤ 0.10 obtained for most copolymers. Whilst the presence of the trifluoromethylphenylene units in the copolymers seemed unfavorable for charge injection and device current, improved spectral purity and stability in both the photoluminescence and electroluminescence were noted and ascribed to the electron-withdrawing nature of the trifluoromethylphenylene units and the enhanced anti-autoxidation ability of the fluorene rings in these copolymers.
In this study, two low bandgap copolymers composed of fluorene (Fl), cyclopentadithiophene (CDT), and 4,7-bis(2-thienyl)-2,1,3-benzothiadiazole (DBT) were synthesized, and their optical, electrochemical, and photovoltaic (PV) characteristics were investigated for applications in PV devices. The feed ratio of the Fl and CDT moieties was modulated to tune the electronic structures and resulting optical properties of the polymers. In the copolymeric structures, the Fl-CDT unit absorbs the short-wavelength UV/vis regions, and the CDT-DBT (or Fl-DBT) unit with strong intramolecular charge transfer characteristics covers the long-wavelength visible regions. P1 exhibited a wide UV absorption spectrum covering the UV and entire visible region in the range of 300-800 nm, and P2 showed absorption covering from 300 to 700 nm. UV/vis and electrochemical studies confirmed the desirable highest occupied molecular orbital/lowest unoccupied molecular orbital levels of the copolymers with bandgaps of 1.62-1.86 eV, enabling efficient electron transfer and a high open-circuit voltage when blending them with fullerene derivatives. When the polymers were blended with [6,6]phenyl-C61-butyric acid methyl ester, P1 exhibited the best device performance with an open-circuit voltage of 0.66 V, short-circuit current of 4.92 mA cm-2, and power conversion efficiency of 1.13% under Air Mass 1.5 Global (AM 1.5G, 100 mW cm-2) illumination.
A series of copolymers, poly[(9,9-dioctylfluorene-2,7-diyl) -co-(4-dicyanomethylene-2-methyl-6-[4-(diphenylamino)styryl]-4H-pyran-4′, 4″-diyl], were synthesized by polymerizing 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-9,9-dioctylfluorene with mixtures of 2,7-dibromo-9,9-dioctylfluorene and 4-dicyanomethylene-2-methyl-6-[bis(4′-bromophenyl)amino]styryl]-4H-pyran (a DCM derivative) by the palladium-catalyzed Suzuki coupling reaction. The copolymers were characterized by molecular weight determination, elemental analysis, 1H NMR, FT-IR spectroscopy, DSC, TGA, UV-vis spectroscopy, and photoluminescence (PL) and electroluminescence (EL) spectroscopy. The copolymers showed two absorption peaks at 380 and 485 nm, and the long-wavelength absorption increased with increasing the fraction of the DCM comonomer. The PL spectra of copolymers in chloroform solution displayed emission from both the main chain (420 nm) and DCM units (620 nm). In the solid state, however, PL spectra of copolymers showed only the long wavelength red emission at 620 nm with no trace of emission from the main chain, which implies a facile exciton migration or energy transfer to the lower energy sites from the fluorene part to the DCM part. This results in emission of only the red light originating from the latter segments. A study on time-resolved PL rise and decay of the polymers clearly supports the energy transfer mechanism. Light-emitting diode (LED) devices were fabricated to have the configuration of ITO (indiumtin oxide)/PEDOT/polymer/Li:Al alloy. EL spectra of the devices showed only red emissions as observed in the PL spectra of the polymers' thin films. EL efficiency decreased with increasing DCM contents. When a tris(8-hydroxyquinolinato)aluminum (Alq3) layer was inserted between the emitting polymer layer and the cathode to make the ITO/PEDOT/polymer/Alq3/Li:Al alloy configuration, the device efficiencies became much higher (~10-2%) than those (5 × 10-5-5 × 10-3%) of single-layer devices.
A new alternating polyfluorene copolymer, poly(9,9′-dioctylfluorene-alt-thieno[3,2-b]thiophene)(PFTT), containing a thiophene-condensed thieno[3,2-b]thiophene moiety has been synthesized via a palladium-catalyzed Suzuki coupling reaction. The synthesized polymer was successfully characterized by 1H NMR, 13C NMR, and elemental analysis. It shows good thermal stability and displays unique phase transition behavior between the crystalline and liquid-crystalline states. The ionization potential and electron affinity of PFTT are -5.38 eV and -2.40 eV, respectively, as determined by cyclic voltammetry. Thus, PFTT has an electrochemical band gap of approximately 2.98 eV, which is smaller than that of common polyfluorene (PF) homopolymers. As a film, PFTT exhibits UV-vis and photoluminescence maxima at 471 and 511 nm, respectively. A light-emitting diode device fabricated with an ITO/PEDOT/PFTT/LiF/Al configuration exhibits pure green light emission with the full width at half-maximum (fwhm) of only 57 nm and a low turn-on voltage of 3.3 V. Especially, this emission has the CIE coordinates (0.29, 0.63), which are very close to the standard for green used by the National Television System Committee. In addition, PFTT exhibits better electroluminescence performance than other similar PF homopolymers and than fluorene- and thiophene-based copolymers.
Two supramolecular systems were constructed based on fluorene-based π-conjugated monomers with or without spiro structures, respectively, and their self-assemble behaviour and optical properties were investigated. Concentration-dependent 1H NMR
A novel acidic poly(fluorenylene) derivative has been synthesized which, upon base-doping, shows electrical conductivities of 10-6-10-5 S cm-1; this new doping method for conjugated polymers opens the way to the preparation of air-stable n-type conducting polymers.
AIE/AIEE-active conjugated polymers have shown great potential in bioimaging applications. However, the absorption of many AIE/AIEE polymers poorly matches with the laser excitation used in confocal imaging, which may greatly affect the imaging quality. I
The present invention relates to compounds of the formulae (1) to (4), which are suitable for use in electronic devices, in particular organic electroluminescent devices, to intermediate compounds for the compounds or formulae (1) to (4) and to electronic devices, which comprise the compounds of formulae (1) to (4).
A series of highly emissive π-conjugated A-alt-B type copolymers (P1-P3) appended with a 1,2,3-triazole moiety was synthesized via Suzuki polymerization. The well-defined and soluble π-conjugated copolymers were characterized via multinuclear NMR spectroscopy and tetradetector GPC studies, showing a molecular weight (Mn) in the range of 16.4-20.1 kDa with a polydispersity index in the range of 1.25-1.42. The synthesized emissive π-conjugated polymer probes were explored as fluorescent chemosensors for nitroaromatic compounds (NACs) in solution, vapor and contact mode. Detailed photophysical and sensing studies were performed to understand the polymer-NAC interaction, inducing the selective fluorescence quenching of the π-conjugated polymer probes through the photoinduced electron transfer (PET) mechanism. All the polymeric probes (P1-P3) were highly reversible in nature with NACs, and thus could be reused multiple times. The limit of detection of the probes towards nitroaromatics was found to be in the range of 120-200 ppb with a high association constant in the order of 104 M-1. Furthermore, test paper kits were also fabricated, which allowed the trace detection of picric acid by the naked eye, making it a practical means for the quick, easy and inexpensive on-site detection of NAC-based explosives.
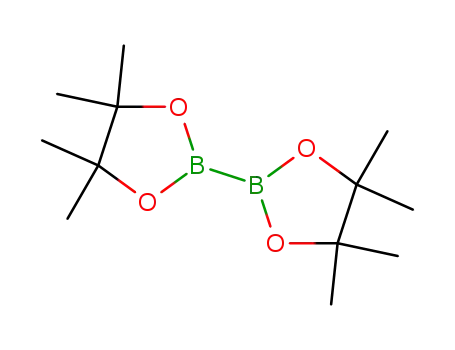
bis(pinacol)diborane

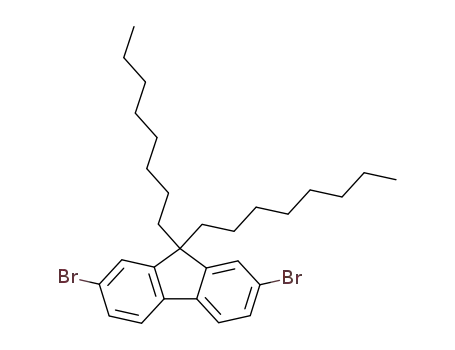
2,7-dibromo-9,9-dioctylfluorene

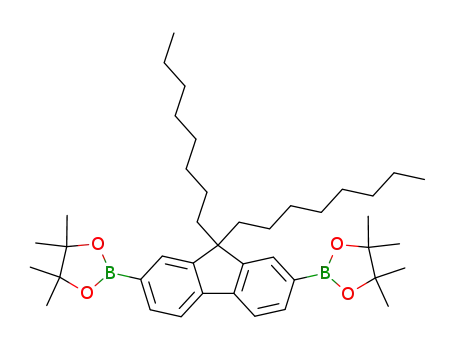
2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-9,9-dioctylfluorene
| Conditions | Yield |
|---|---|
|
With
(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate;
In
1,4-dioxane;
at 80 ℃;
Inert atmosphere;
|
91% |
|
With
(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate;
In
1,4-dioxane;
at 100 ℃;
for 18h;
Inert atmosphere;
|
83% |
|
With
dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate;
In
N,N-dimethyl-formamide;
at 90 ℃;
for 4h;
Inert atmosphere;
|
72% |
|
With
(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate;
In
N,N-dimethyl-formamide;
at 80 ℃;
for 10h;
Glovebox;
|
72% |
|
With
(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate;
In
1,4-dioxane; water;
at 80 ℃;
Inert atmosphere;
|
72% |
|
With
(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate;
at 105 ℃;
Inert atmosphere;
|
70% |
|
With
(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate;
In
1,4-dioxane;
at 90 ℃;
for 6h;
Inert atmosphere;
|
63% |
|
With
bis-triphenylphosphine-palladium(II) chloride; triphenylphosphine;
In
toluene;
at 110 ℃;
for 24h;
Inert atmosphere;
|
13% |
|
With
(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate;
In
dimethyl sulfoxide;
|
|
|
With
dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate;
In
N,N-dimethyl-formamide;
|
|
|
With
(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate;
In
1,4-dioxane;
|
|
|
With
potassium acetate; palladium diacetate;
In
1,4-dioxane;
at 85 ℃;
for 8h;
|
|
|
With
(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate;
In
1,4-dioxane;
at 110 ℃;
Reflux;
Inert atmosphere;
|
|
|
With
(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate;
In
dimethyl sulfoxide;
at 65 ℃;
|
|
|
With
(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate;
In
1,4-dioxane; toluene;
|
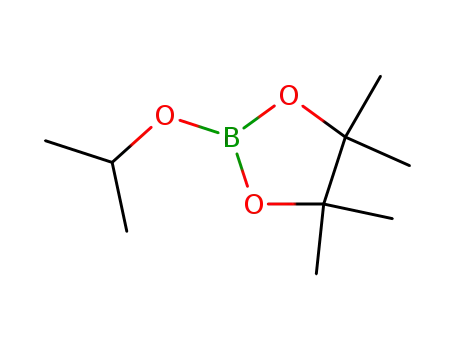
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane


2,7-dibromo-9,9-dioctylfluorene


2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-9,9-dioctylfluorene
| Conditions | Yield |
|---|---|
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 - 0 ℃;
for 0.616667h;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
for 30h;
|
94% |
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 2h;
Inert atmosphere;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran; hexane;
at 20 ℃;
for 20h;
Inert atmosphere;
|
80% |
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 2h;
Inert atmosphere;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 1h;
|
80% |
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 2h;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran;
at -78 - 20 ℃;
for 50h;
|
78% |
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 0.75h;
Schlenk technique;
Inert atmosphere;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran; hexane;
at -78 - 28 ℃;
for 24h;
Schlenk technique;
Inert atmosphere;
|
78% |
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 2h;
Inert atmosphere;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran; hexane;
at 20 ℃;
for 20h;
|
77% |
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 1h;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
|
74% |
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 1.5h;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
|
74% |
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 1h;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran; hexane;
at 20 ℃;
|
74% |
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 2h;
Inert atmosphere;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran;
at -78 - 20 ℃;
for 25h;
|
70% |
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 2h;
Inert atmosphere;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran;
at -78 - 20 ℃;
for 25h;
Inert atmosphere;
|
70% |
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 2h;
Inert atmosphere;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran;
at -78 - 20 ℃;
for 25h;
|
70% |
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 2h;
Inert atmosphere;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran;
at -78 - 20 ℃;
for 25h;
|
70% |
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 2h;
Inert atmosphere;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran;
at -78 - 20 ℃;
for 25h;
Inert atmosphere;
|
70% |
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 2h;
Inert atmosphere;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran;
at -78 - 20 ℃;
for 36.6667h;
Inert atmosphere;
|
70% |
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 2h;
Inert atmosphere;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran;
at -78 - 20 ℃;
for 25h;
|
70% |
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 2h;
Inert atmosphere;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran;
at -78 ℃;
for 1h;
Inert atmosphere;
|
70% |
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 2h;
Inert atmosphere;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran;
at -78 - 20 ℃;
for 25h;
Inert atmosphere;
|
70% |
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 2h;
Inert atmosphere;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran;
at -78 - 20 ℃;
for 25h;
|
70% |
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 1h;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
|
68% |
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 2h;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
for 17.5h;
|
66% |
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 - 0 ℃;
for 0.25h;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran; hexane;
at 20 ℃;
for 24h;
|
65% |
|
With
n-butyllithium;
In
tetrahydrofuran;
react. of fluorene with BuLi at -78°C, then with dioxaborolane;
|
65% |
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 2h;
Inert atmosphere;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
at -78 - 20 ℃;
for 14h;
Inert atmosphere;
|
65% |
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 2h;
Inert atmosphere;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran;
at -78 - 20 ℃;
for 14h;
Inert atmosphere;
|
65% |
|
With
tert.-butyl lithium;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
|
59% |
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 1h;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
Further stages.;
|
57% |
|
2,7-dibromo-9,9-dioctylfluorene;
With
tert.-butyl lithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 2h;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
for 1.66667h;
|
56% |
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 1h;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran;
at -78 - 20 ℃;
for 2h;
Further stages.;
|
55% |
|
2,7-dibromo-9,9-dioctylfluorene;
With
tert.-butyl lithium;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
for 4h;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
for 41h;
|
52.7% |
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 0.5h;
Inert atmosphere;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
|
50% |
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 2h;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
for 38h;
|
46% |
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 2h;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
for 38h;
|
46% |
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
at -78 ℃;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
|
46% |
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 2h;
Inert atmosphere;
Darkness;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
for 12h;
Inert atmosphere;
Darkness;
|
42% |
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 1.33333h;
Inert atmosphere;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran;
at 20 ℃;
Inert atmosphere;
|
34.9% |
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 - 20 ℃;
for 0.25h;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran;
at -78 - 20 ℃;
for 24h;
|
|
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran;
|
|
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran;
|
|
|
2,7-dibromo-9,9-dioctylfluorene;
With
lithium;
In
tetrahydrofuran;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran;
|
|
|
With
n-butyllithium;
at -78 ℃;
|
|
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 2h;
Inert atmosphere;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran;
for 25h;
|
|
|
With
tert.-butyl lithium;
In
tetrahydrofuran;
at -78 ℃;
|
|
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran;
|
|
|
2,7-dibromo-9,9-dioctylfluorene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
Inert atmosphere;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran;
Inert atmosphere;
|
|
|
With
sec.-butyllithium;
In
tetrahydrofuran;
|
|
|
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
|
|
|
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
|

2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane

2,7-dibromo-9,9-dioctylfluorene
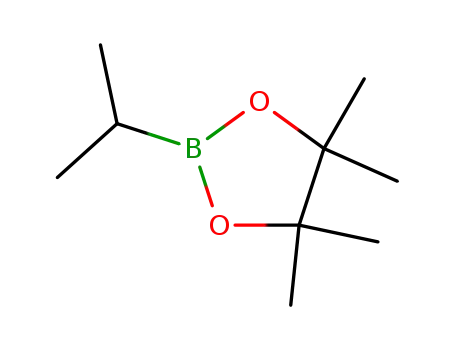
2-isopropyl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
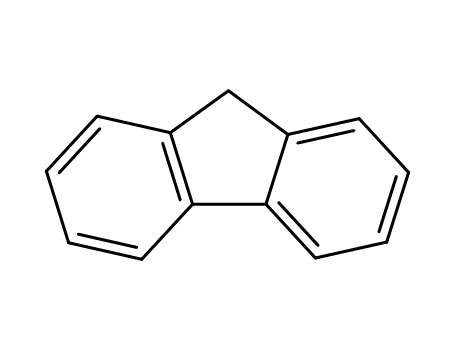
9H-fluorene
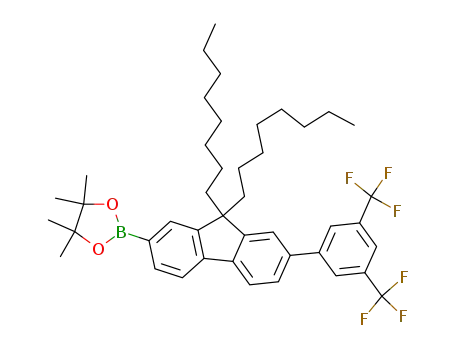
2-{7-[3,5-bis(trifluoromethyl)phenyl]-9,9-dioctyl-9H-fluoren-2-yl}-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
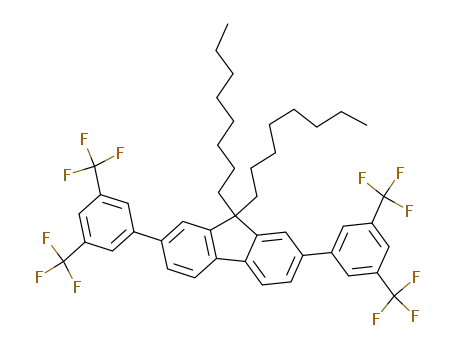
2,7-bis[3,5-bis(trifluoromethyl)phenyl]-9,9-dioctyl-9H-fluorene
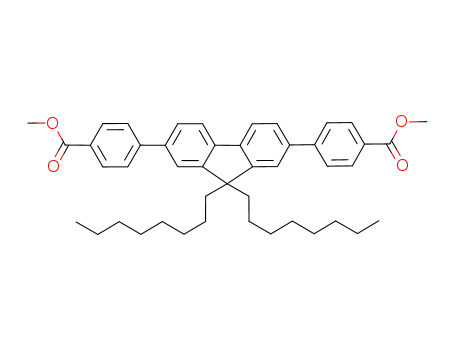
2,7-bis(4-phenylcarboxylic acid methyl ester)-9,9-di-n-octylfluorene

2,7-bis(4-phenylcarboxylic acid)-9,9-di-n-octylfluorene
CAS:955959-91-8
CAS:80-04-6
Molecular Formula:C<sub>15</sub>H<sub>28</sub>O<sub>2</sub>
Molecular Weight:240.38
CAS:439791-57-8