Your Location:Home >Products >OLED intermediates >Fluorenes >439791-57-8

Product Details
Abstract A tert-butylated spirobifluorene derivative incorporating triphenylamine groups was synthesized starting from the readily available reagent biphenyl. Without any hole-transport layer, an unoptimized double-layer device exhibits excellent electroluminescent performances with a radiance of 3013 cd/m2 at 9.8 V, a maximum electroluminescent efficiency of 1.71 cd/A, a maximal external quantum efficiency of 2.58% at a brightness of 4.8 cd/m2, narrow full width at half-maximum (54 nm), and blue emission with Commission Internationale de l'Eclairage coordinates of (0.152, 0.103), which is close to the standard for blue. Compared to the reported organic light emitting diodes materials, the molecule displays very high thermal stability. In addition, the bilayer device showed a greatly improved performance as compared to a trilayer device with NPB as hole-transporting layer, which indicated that the molecule possesses good hole transport ability and can be good candidate for hole-transporting layer in organic light emitting diodes.
Five novel spirobifluorene derivatives, SPF-DM, SPF-MB, SPF-BB, BSPF-NA1 and BSPF-NA2 were synthesized starting from the readily available reagent 4,4′-bisalkylated biphenyl. Their linear absorption, fluorescence and thermal properties were examined and their redox potential levels were estimated by cyclic voltammetry. These compounds possess high thermal stability. SPF-MB and SPF-BB possess excellent spectral sensitivities to pH changes and can be used as optical pH sensors of a wide pH range. The recognition behavior of SPF-BB toward various metal ions has been evaluated in a acetonitrile/water (15:1, v/v) solvent system. SPF-BB is highly selective in recognizing Ag +. The electroluminescent properties of SPF-DM, SPF-MB, BSPF-NA1 and BSPF-NA2 are demonstrated with a device configuration of ITO/MoO3 (6 nm)/NPB (80 nm)/EML (30 nm)/TPBI (40 nm)/LiF (1 nm)/Al (100 nm). The devices of BSPF-NA1 and BSPF-NA2 display a yellow emission in the EL spectra and their maximum brightness reached 380 and 311 cd m-2, respectively. At low turn-on voltage (7.9 V) a light blue emission was observed in the device of SPF-DM and its maximum brightness reached 391 cd m-2. Notably, the device of SPF-MB has a white emission with CIE coordinates (0.293, 0.307).
The novel spirobifluorene diamine monomer, 2,7-bis-amino-2′,7′- di-tert-butyl-9,9′-spirobifluorene, was obtained. The new organosoluble polyimides were prepared from spirobifluorene diamine containing bulky tert-butyl group and conventional dianhydrides as well as 2,2- ′bis(4″-tert-butylphenyl)4,4′,5,5′- biphenyltetracarboxylic dianhydride (BBBPAn) and 2,2′-bis(4″- trimethylsilylphenyl)-4,4′,5,5′biphenyltetracarboxylic dianhydride (BTSBPAn), which are composed of a noncoplanar twisted biphenyl unit containing bulky p-tert-butylphenyl groups and/or p-trimethylsilylphenyl groups by high-temperature one step polymerization. The structures of polymers were confirmed by various spectroscopic techniques. The weight-average molecular weights and polydispersities of resulting polymers were in the ranges 48 300-183 900 and 2.10-3.26, respectively. The bulky and twisted noncoplanar structural feature confers an enhanced solubility of the polyimides because of a decrease in the degree of molecular packing and crystallinity while imparting a significant increase in both Tg and thermal stability by restricting segmental mobility. The new polyimides exhibit the high oxygen permeabilities (P(O2) = 18-121 barrer) and O2/N2 gas separation properties (P(O2)/P(N2) = 2.2-9).
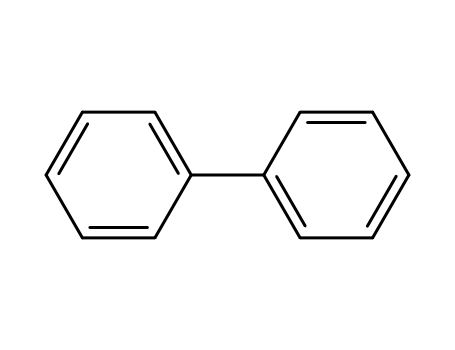
biphenyl

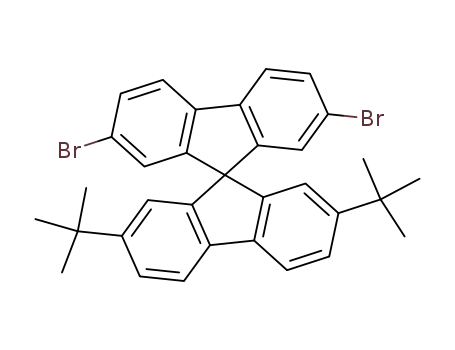
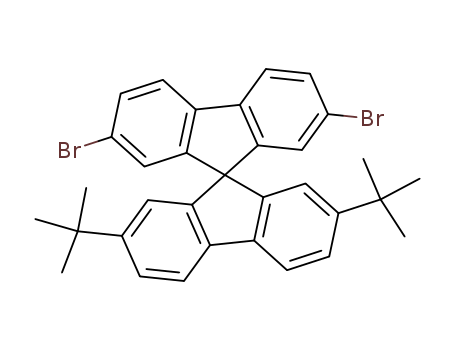
2,7-dibromo-(2',7'-di-tert-butyl)-9,9'-spirobifluorene
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1.1: 80 percent / AlCl3 / nitromethane
2.1: magnesium / diethyl ether / 2 h / Heating
2.2: diethyl ether / 12 h / Heating
3.1: 7 g / hydrochloric acid; acetic acid / H2O / Heating
With
hydrogenchloride; aluminium trichloride; magnesium; acetic acid;
In
diethyl ether; nitromethane; water;
|
|
|
Multi-step reaction with 3 steps
1: aluminum (III) chloride
2: bromine; iodine
3: magnesium / tetrahydrofuran
With
aluminum (III) chloride; bromine; iodine; magnesium;
In
tetrahydrofuran;
|
|
|
Multi-step reaction with 3 steps
1: aluminum (III) chloride
2: bromine; iodine
3: iodine; magnesium / tetrahydrofuran
With
aluminum (III) chloride; bromine; iodine; magnesium;
In
tetrahydrofuran;
|
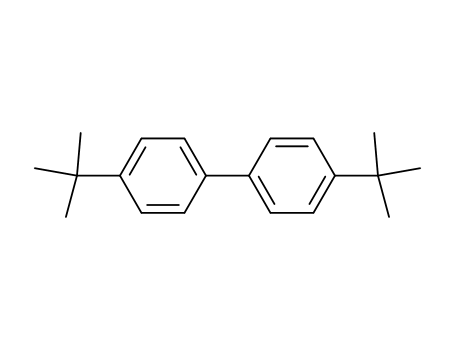
4,4'-di-tert-butylbiphenyl


2,7-dibromo-(2',7'-di-tert-butyl)-9,9'-spirobifluorene
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1.1: magnesium / diethyl ether / 2 h / Heating
1.2: diethyl ether / 12 h / Heating
2.1: 7 g / hydrochloric acid; acetic acid / H2O / Heating
With
hydrogenchloride; magnesium; acetic acid;
In
diethyl ether; water;
|
|
|
Multi-step reaction with 2 steps
1: bromine; iodine
2: magnesium / tetrahydrofuran
With
bromine; iodine; magnesium;
In
tetrahydrofuran;
|
|
|
Multi-step reaction with 2 steps
1: bromine; iodine
2: iodine; magnesium / tetrahydrofuran
With
bromine; iodine; magnesium;
In
tetrahydrofuran;
|

biphenyl
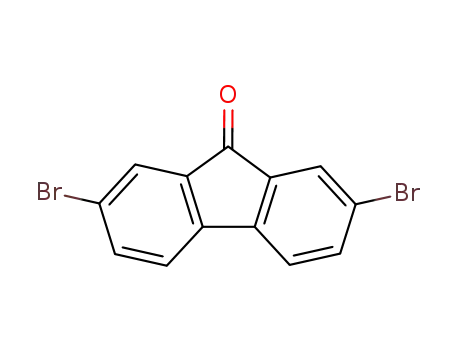
2,7-dibromofluorene-9-one

4,4'-di-tert-butylbiphenyl

2-bromo-4,4'-di-tert-butylbiphenyl
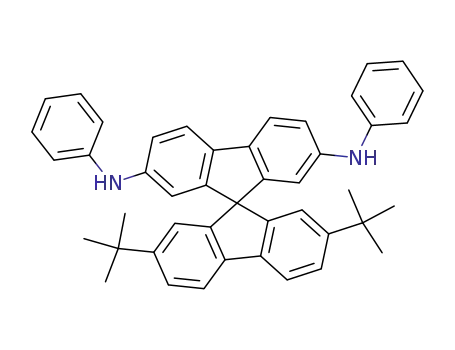
N,N'-diphenyl-[(2',7'-di-tert-butyl)-9,9'-spirobifluorene]-2,7-diamine

2-(3,5-di-tert-butylphenyl)-7-(4-diphenylaminostyryl)-2',7'-di-tert-butyl-9,9'-spirobifluorene

2-(p-triphenylmethylphenyl)-7-(4-diphenylaminostyryl)-2',7'-di-tert-butyl-9,9'-spirobifluorene

2-(p-triphenylsilylphenyl)-7-(4-diphenylaminostyryl)-2',7'-di-tert-butyl-9,9'-spirobifluorene
CAS:610-09-3
Molecular Formula:C<sub>8</sub>H<sub>12</sub>O<sub>4</sub>
Molecular Weight:172.18
CAS:333432-28-3
Molecular Formula:C15H15BO2
Molecular Weight:238.09
CAS:319906-45-1
Molecular Formula:C15H12BrI
Molecular Weight:399.06