Your Location:Home >Products >OLED intermediates >Fluorenes >2041-19-2

Product Details
Cyclisation of radicals 6a,b is highly regioselective towards a 5-exo process; 6-endo ring closure is a minor route and their ratio depends on the substituents.No ring expansion of the five-membered radical intermediates 7a,b was observed.Radicals 27a,b give rise to 5-exo cyclisation regiospecifically.A competitive 1,5-hydrogen shift leading to imidoyl radicals was noticed.An analogous behaviour is also exhibited by vinyl radicals when allowed to add to carbon-nitrogen double bonds.
The title reactions have been investigated in aq. acetic acid medium both in the presence and absence of perchloric acid.The reactions are first order each in and under both the conditions.There is unit dependence in perchloric acid.The effects of change in polarity of the solvent medium, added toluene-p-sulphonamide and sodium perchlorate have been studied.The reaction exhibits kinetic isotope effect (kH/kD = 2.2).Hammett ρ is found to be -2.8.Activation parameters have been evaluated.A mechanism consistent with rate data has been proposed.
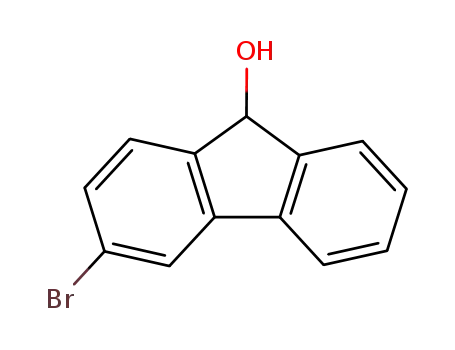
3-bromofluoren-9-ol

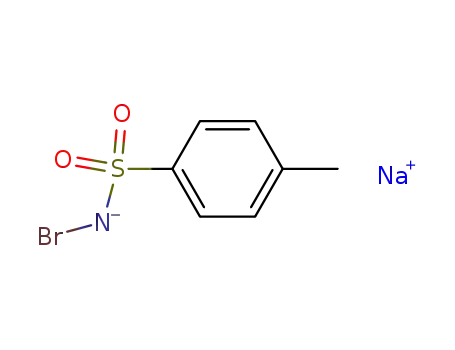
bromamine T

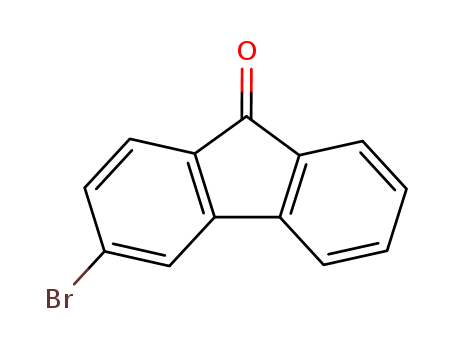
3-bromofluoren-9-one

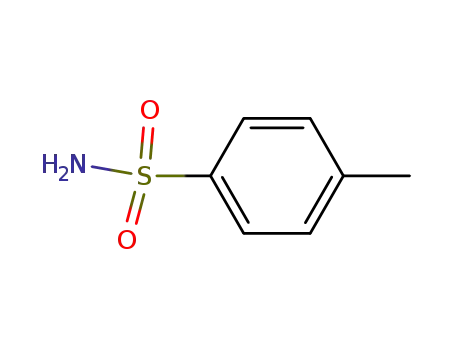
toluene-4-sulfonamide
| Conditions | Yield |
|---|---|
|
With
acetic acid;
for 24h;
|
|
|
With
acetic acid;
at 30 ℃;
Kinetics;
Mechanism;
Thermodynamic data;
E(activ.), ΔH(activ.), ΔS(activ.), Γ(activ.);
|
N-<(5'-bromo-2'-iodobiphenyl-2yl)methylene>-4-chlorobenzenamine


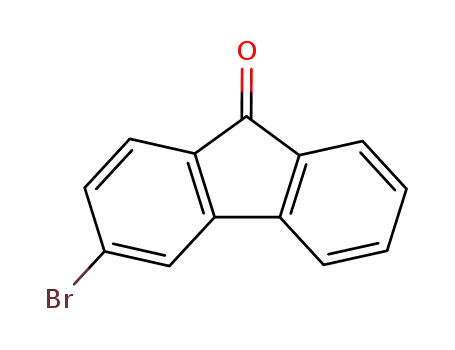
3-bromofluoren-9-one

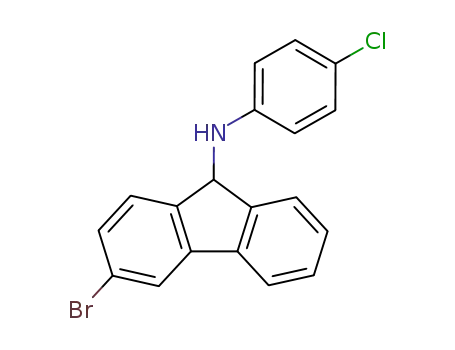
N-(3-bromo-9H-fluoren-9-yl)-4-chlorobenzenamine
| Conditions | Yield |
|---|---|
|
With
2,2'-azobis(isobutyronitrile); tri-n-butyl-tin hydride;
Yield given. Multistep reaction;
1.) benzene, reflux, 7 h; 2.) column chromatography;
|
50% |
|
With
2,2'-azobis(isobutyronitrile); tri-n-butyl-tin hydride;
In
benzene;
for 7h;
Heating;
|
5% 50% |

3-bromofluoren-9-ol

bromamine T
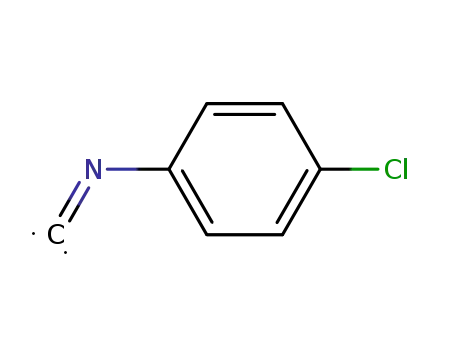
p-chlorophenyl isocyanide
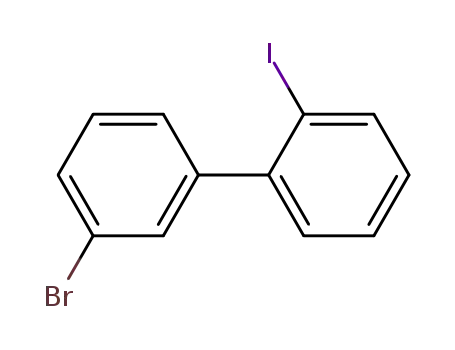
3-bromo-2'-iodobiphenyl

3-bromofluoren-9-ol
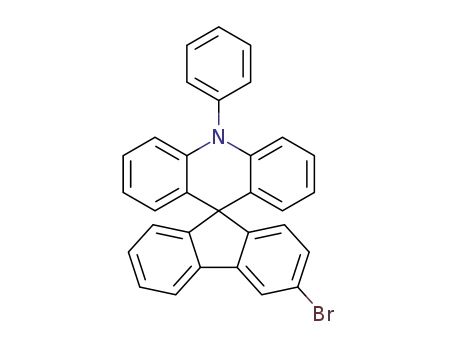
3'-bromo-10-phenyl-10H-spiro(acridine-9,9'-fluorene)
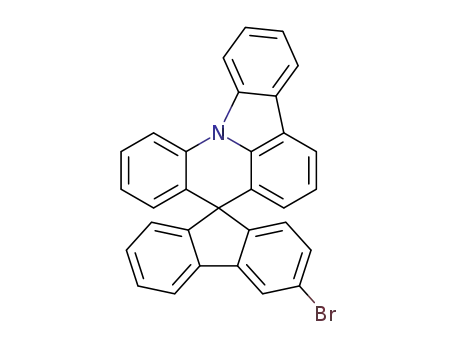
3-bromospiro[fluorene-9,8'-indolo[3,2,1-de]acridine]
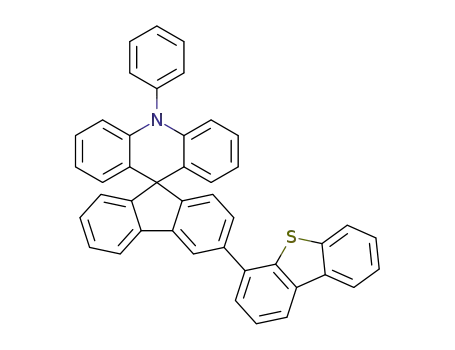
C43H27NS
CAS:2746-19-2
Molecular Formula:C9H8O3
Molecular Weight:164.16
CAS:16807-13-9
CAS:402936-15-6
CAS:1674334-51-0