Your Location:Home >Products >OLED intermediates >Fluorenes >1674334-51-0

Product Details
We have developed a one-pot synthesis of benzo[b]fluorenones via a cobalt-catalyzed [3+2] annulation of oxabicyclic alkenes followed by a ring-opening/dehydration sequence in good to excellent yields. With the use of 2-(1-methylhydrazinyl)pyridine (MHP),
Provided is an anthracene derivative represented by formula (1). In formula (1), one of R11 to R20 is used to bond to L1, and those of R11 to R20 not used to bond to L1 are each independently a hydrogen atom, a halogen atom, a cyano group, a substituted o
The invention provides a composition, a film, an optical device, and a compound that can form a film having a high refractive index and exhibits excellent developability and curability.? The composition of the invention includes a compound represented by the following general formula (1). In the general formula (1), Ar1 -Ar4 each represent an aromatic ring group independently.? At least one of Ar1 -Ar4 is a polycyclic aromatic group and satisfies the following requirement 1 or requirement 2. Requirement 1: At least one of Ar1 -Ar4 has a substituent containing an acid group and a polymerizable group. Requirement 2: At least one of Ar1 -Ar4 has a substituent containing an acid group and at least one of Ar1 -Ar4 has a substituent containing a polymerizable group.
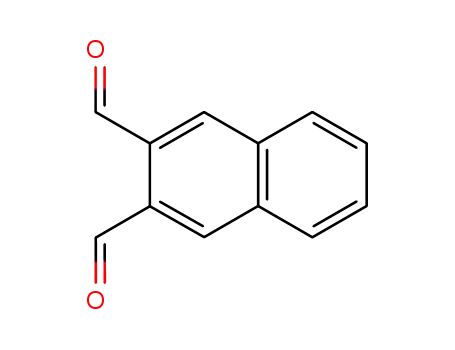
2,3-naphthalenedicarboxaldehyde

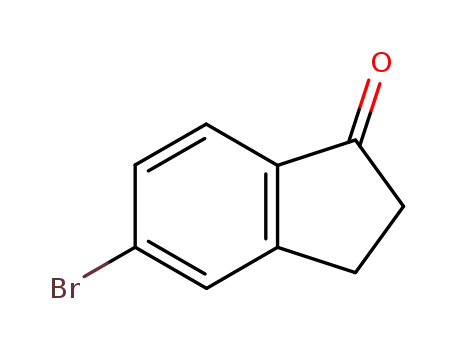
5-Bromo-1-indanone

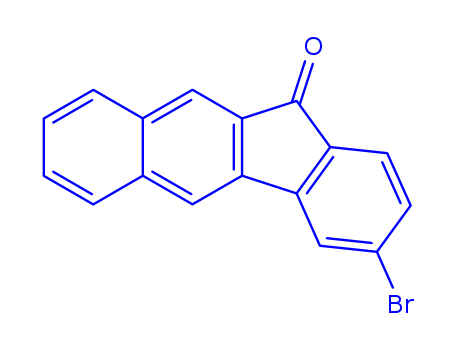
![3-bromobenzo[b]fluorene-11-one](/upload/2023/2/5bdd3d74-4f1d-4fc3-9f86-f09d3c287d0c.png)
3-bromobenzo[b]fluorene-11-one
| Conditions | Yield |
|---|---|
|
With
sodium ethanolate;
In
ethanol;
at 20 ℃;
for 32h;
Reflux;
|
74 g |

5-Bromo-1-indanone

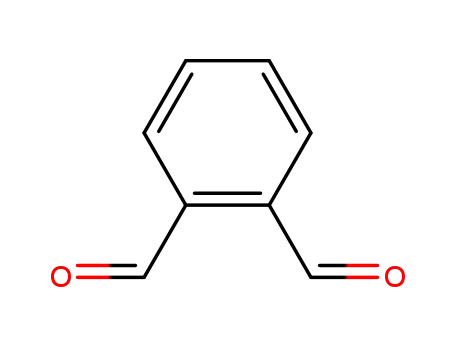
o-phthalic dicarboxaldehyde

![3-bromobenzo[b]fluorene-11-one](/upload/2023/2/5bdd3d74-4f1d-4fc3-9f86-f09d3c287d0c.png)
3-bromobenzo[b]fluorene-11-one
| Conditions | Yield |
|---|---|
|
With
potassium hydroxide;
In
methanol;
at 30 - 55 ℃;
for 2h;
|
37 g |

2,3-naphthalenedicarboxaldehyde

5-Bromo-1-indanone
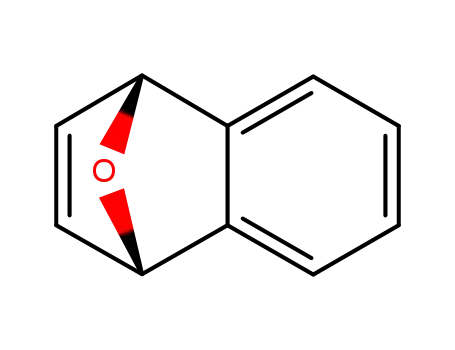
1,4-epoxy-1,4-dihydronaphthalene
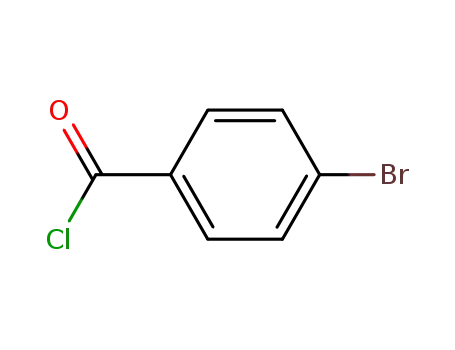
4-chlorobenzoyl chloride
CAS:1001911-63-2
Molecular Formula:C<sub>18</sub> H<sub>14</sub> BNO<sub>2</sub>
Molecular Weight:287.1
CAS:2041-19-2
CAS:867374-53-6