Your Location:Home >Products >OLED intermediates >Fluorenes >867374-53-6
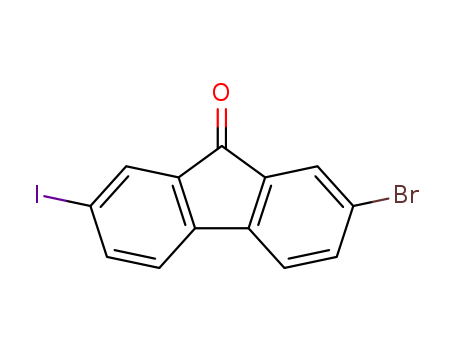

Product Details
A series of amphiphilic PEG-functionalized nitrogen ligands were developed for the highly efficient copper-catalyzed aerobic oxidation of 9-fluorenes, with molecular oxygen as the sole oxidant in neat water. A broad range of functional groups are well tolerated and thus offer the opportunity for further functionalization.
Aiming at the technical problems that in the prior art a method for synthesizing a fluorenone ketone compound has organic solvent pollution and byproducts can be generated, the invention provides a method for synthesizing a fluorenone ketone compound through molecular oxygen oxidation in a water phase. The method comprises the following steps: by taking a fluorenone compound as a substrate, dispersing into an alkali solution, and at 40-120 DEG C, in the presence of oxygen, and with a water-soluble transition metal compound as a catalyst, stirring to carry out reactions, thereby obtaining the fluorenone ketone compound. By adopting the method, molecular oxygen is adopted as an oxidant, and water is adopted as a solvent, so that an organic solvent is avoided, and the problem that multiple byproducts are generated because of peroxidation can be avoided.
An efficient direct C(sp3)–H oxidation of diarylmethanes has been demonstrated by this study. This method employs environment-friendly O2 as an oxidant and is promoted by commercially available MN(SiMe3)2 [M = K, Na or Li], which provides a facile method for the synthesis of various diaryl ketones in excellent yields. This protocol is metal-free, mild and compatible with a number of functional groups on substrates.
We developed a simple and efficient Cu(II)-catalyzed ligand-free oxidation of diarylmethanes and secondary alcohols using 70% tert-butyl hydroperoxide (TBHP) in water. A series of diarylmethanes were directly oxidized into diaryl ketones in 67%–98% yields. Additionally, various secondary alcohols were also transformed into the desired products in 48%–98% yields. Importantly, the catalytic system in the absence of any organic solvent, surfactant, or phase transfer agent, had a wide substrate scope and a high tolerance for various functional groups.
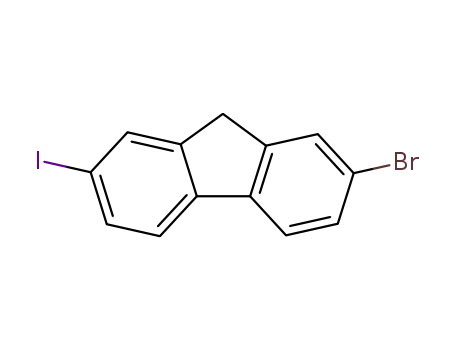
2-bromo-7-iodofluorene

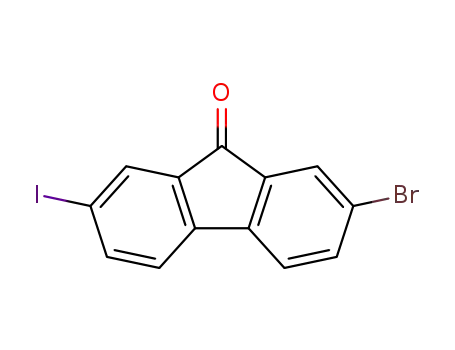
2-bromo-7-iodo-9H-fluoren-9-one
| Conditions | Yield |
|---|---|
|
With
copper(II) choride dihydrate; 4,7-bis(2-(2-(2-methoxyethoxy)ethoxy)ethoxy)-1,10-phenanthroline; oxygen; sodium hydroxide;
In
water;
at 50 ℃;
for 16h;
Schlenk technique;
|
93% |
|
With
potassium phosphate; C38H60N2O12; oxygen; palladium dichloride;
In
water;
at 70 ℃;
for 24h;
under 1500.15 Torr;
|
93% |
|
With
oxygen; lithium hexamethyldisilazane;
In
tetrahydrofuran;
at 60 ℃;
for 12h;
under 760.051 Torr;
Sealed tube;
Green chemistry;
|
87% |
|
With
chromium(VI) oxide; acetic anhydride; acetic acid;
at 20 ℃;
for 16h;
|
85% |
|
With
chromium(VI) oxide;
In
acetic anhydride; acetic acid;
at 20 ℃;
for 16h;
Inert atmosphere;
|
73% |
|
With
tert.-butylhydroperoxide; copper(II) acetate monohydrate;
In
water;
at 80 ℃;
for 8h;
|
67% |
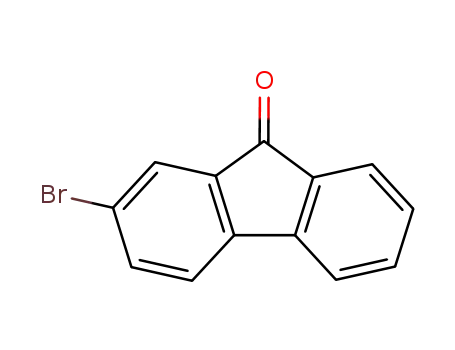
2-bromofluoren-9-one


2-bromo-7-iodo-9H-fluoren-9-one
| Conditions | Yield |
|---|---|
|
2-bromofluoren-9-one;
With
sulfuric acid; acetic acid;
In
water;
at 60 ℃;
for 1h;
With
iodine; periodic acid;
In
water;
at 85 ℃;
for 16h;
|
90% |
|
2-bromofluoren-9-one;
With
sulfuric acid;
In
water; acetic acid;
at 60 ℃;
for 1h;
Inert atmosphere;
With
iodine; periodic acid;
In
water; acetic acid;
at 85 ℃;
for 18h;
regioselective reaction;
Inert atmosphere;
|
89% |
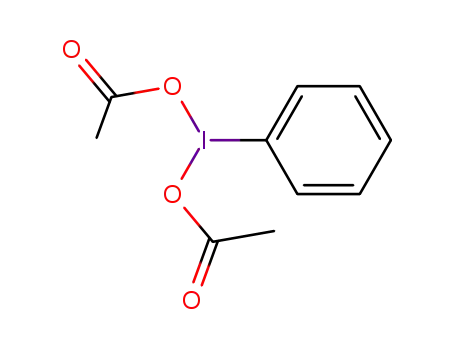
[bis(acetoxy)iodo]benzene
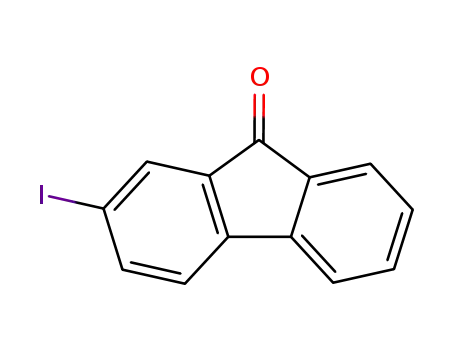
2-iodofluorenone

2-bromo-7-iodofluorene

2-bromo-9H-fluorene
CAS:1210469-11-6
Molecular Formula:C22H14BrN
Molecular Weight:372.3
CAS:1372778-66-9
Molecular Formula:C27H23N
Molecular Weight:361.5
CAS:1674334-51-0
CAS:319906-45-1
Molecular Formula:C15H12BrI
Molecular Weight:399.06