Your Location:Home >Products >Functional intermediates >755017-61-9

Product Details
The enzymatic acylative desymmetrization of σ-symmetric 2'-halo-1,1'-biphenyl-2,6-diols was achieved for the first time using commercially available Burkholderia cepacia lipase immobilized on diatomaceous earth to give (S)-mono esters. The hydrolytic desymmetrization of the corresponding diacetates was also achieved using the same lipase to give (R)-mono esters. Our results, therefore, demonstrate that a single lipase can conduct the enantiodivergent synthesis of axially chiral biphenyl compounds in high chemical and optical yields.
Teaming up: The important indoline scaffold is provided with enantiomeric ratios of up to 98:2 in palladium(0)-catalyzed C(sp3)-H activations of aryl triflates. The key is the combination of the electron-rich monodentate Sagephos and the bulky 9H-xanthene-9-carboxylic acid. Both participate in a highly cooperative manner in the enantiodetermining concerted-deprotonation- metalation step (see scheme, Tf=triflate). Copyright
Here we describe the development of biaryl 2,5-diphenylphospholanes as a new class of C2-symmetric, monodentate ligands for asymmetric Suzuki-Miyaura (ASM) reactions. Screening of a series of exemplary phospholanes led to the identification of two ligands that were used to prepare a range of atropisomeric biaryl and heterobiaryl products with good to excellent levels of enantioselectivity (up to 97:3 e.r.) under mild conditions. DFT studies suggest that the formation of a constraining ligand pocket and coordination of one of the biaryl methoxy groups in the optimised ligands to the metal centre is crucial for restricting conformational freedom in the bond-forming step. (Figure presented.).
Axially chiral phenols are attractive targets in organic synthesis. This motif is central to many natural products and widely used as precursors to, or directly, as chiral ligands and catalysts. Despite their utility few simple catalytic methods are avail
The rational design and synthesis of a novel dialkylbiarylphosphine ligand, 2′-(dimethylphosphine)-2,6-dimethoxy-1,1′-biphenyl (MeSPhos), for palladium-catalyzed C-N cross-coupling reactions is described. Based on previous results, it was hypothesized that a ligand with electronic properties similar to (2-biphenyl)dimethylphosphine (MeJPhos) but with greater steric bulk would allow the cross-coupling of previously inaccessible deactivated aryl chlorides. As predicted, MeSPhos exhibited similar electronic properties to MeJPhos. However, MeSPhos surprisingly showed a significantly smaller steric profile than MeJPhos. In comparison to the widely used CySPhos (2-dicyclohexylphosphino-2′,6′-dimethoxybiphenyl), MeSPhos promoted the oxidative addition of highly deactived aryl chlorides for which CySPhos was ineffective, but significantly decreased the rate of reductive elimination. The kinetics of cross-coupling reactions showed that the altered steric and electronic parameters of MeSPhos had a significant impact on the rate of cross-coupling, and the decreased steric bulk had a profound deleterious impact on the catalyst stability. With regard to this latter point, only the most activated aryl chlorides reacted at a sufficient rate to overcome the rate of catalyst decomposition. These results indicate that the relationship between the electron-donating ability of the phosphine ligand and the rate of oxidative addition is complex, and they also illustrate that increasing substitution on the biphenyl structure does not necessarily increase the steric bulk of the ligand.
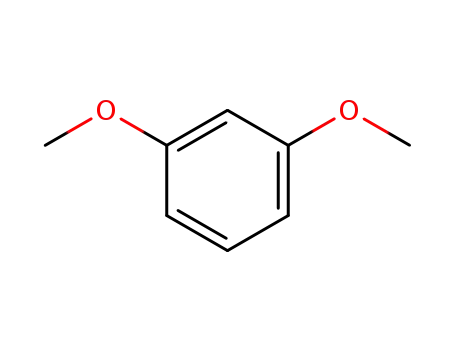
1,3-Dimethoxybenzene

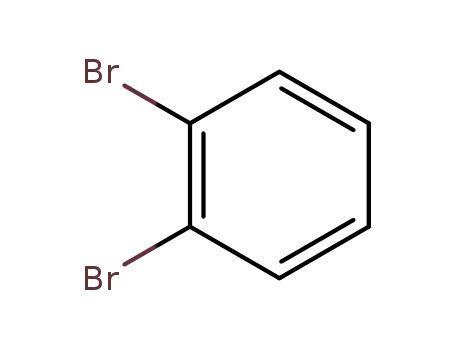
2,3-dibromobenzene

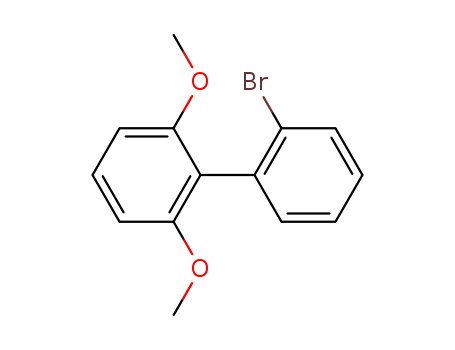
1-bromo-2,6-dimethoxybenzene

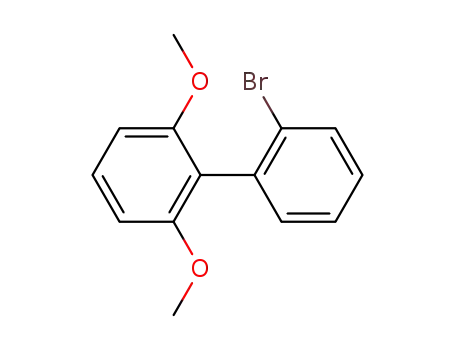
2'-bromo-2,6-dimethoxybiphenyl
| Conditions | Yield |
|---|---|
|
1,3-Dimethoxybenzene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at 0 - 25 ℃;
for 5h;
Inert atmosphere;
2,3-dibromobenzene;
In
tetrahydrofuran; hexane;
at 0 - 25 ℃;
Inert atmosphere;
|
59% 9 %Spectr. |
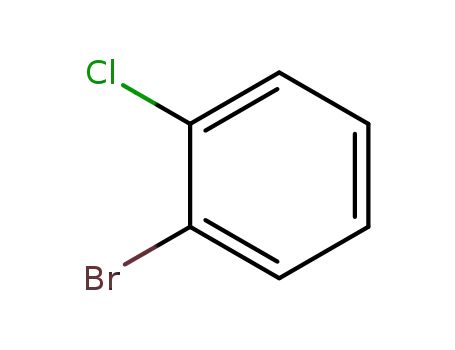
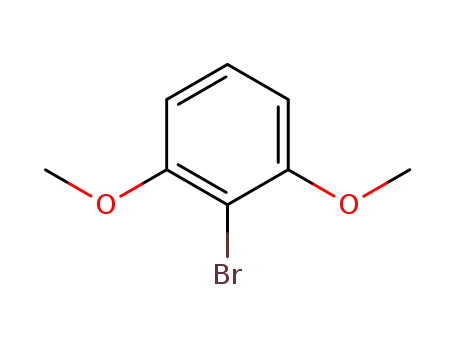
2-bromo-1-chlorobenzene


1,3-Dimethoxybenzene


2'-bromo-2,6-dimethoxybiphenyl
| Conditions | Yield |
|---|---|
|
1,3-Dimethoxybenzene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at 20 ℃;
for 5h;
2-bromo-1-chlorobenzene;
In
tetrahydrofuran; hexane;
at 0 ℃;
for 0.5h;
|
81% |
|
1,3-Dimethoxybenzene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at 0 - 20 ℃;
for 5h;
2-bromo-1-chlorobenzene;
In
tetrahydrofuran; hexane;
at 0 ℃;
for 0.25h;
|
81% |
|
1,3-Dimethoxybenzene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at 0 - 20 ℃;
for 2h;
Inert atmosphere;
2-bromo-1-chlorobenzene;
In
tetrahydrofuran; hexane;
at 0 ℃;
for 0.5h;
Inert atmosphere;
|
77% |
|
1,3-Dimethoxybenzene;
With
n-butyllithium;
In
tetrahydrofuran;
at 0 - 20 ℃;
for 1.75h;
2-bromo-1-chlorobenzene;
In
tetrahydrofuran;
at 0 ℃;
for 0.5h;
|
76% |
|
1,3-Dimethoxybenzene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at 20 ℃;
for 1h;
2-bromo-1-chlorobenzene;
In
tetrahydrofuran; hexane;
at 20 ℃;
for 3h;
Further stages.;
|
70% |
|
1,3-Dimethoxybenzene;
With
n-butyllithium;
In
tetrahydrofuran;
at 0 - 20 ℃;
for 6.16667h;
Inert atmosphere;
Cooling with ice;
2-bromo-1-chlorobenzene;
In
tetrahydrofuran;
at 0 - 20 ℃;
for 12.5h;
Inert atmosphere;
Cooling with ice;
|
58% |
|
1,3-Dimethoxybenzene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at 0 - 25 ℃;
Inert atmosphere;
2-bromo-1-chlorobenzene;
In
tetrahydrofuran; hexane;
Inert atmosphere;
|
57% |
|
1,3-Dimethoxybenzene;
With
n-butyllithium;
In
tetrahydrofuran;
at -5 - 20 ℃;
Inert atmosphere;
2-bromo-1-chlorobenzene;
In
tetrahydrofuran;
at 0 ℃;
for 0.75h;
Inert atmosphere;
|
49% |
|
1,3-Dimethoxybenzene;
With
n-butyllithium;
In
hexane;
Inert atmosphere;
2-bromo-1-chlorobenzene;
In
hexane;
Inert atmosphere;
|
|
|
1,3-Dimethoxybenzene;
With
n-butyllithium;
In
tetrahydrofuran;
2-bromo-1-chlorobenzene;
|
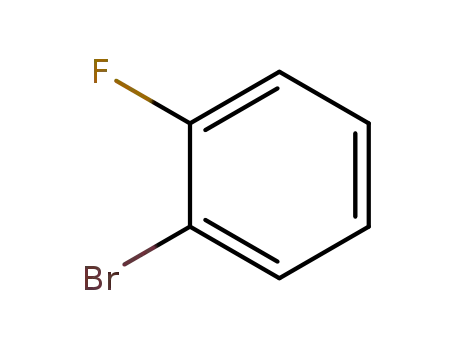
o-fluorobromobenzene

1,3-Dimethoxybenzene

2-bromo-1-chlorobenzene

2,3-dibromobenzene
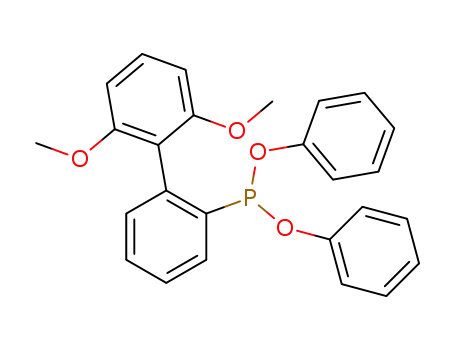
diphenyl 2-(2',6'-dimethoxybiphenylyl)phosphonite
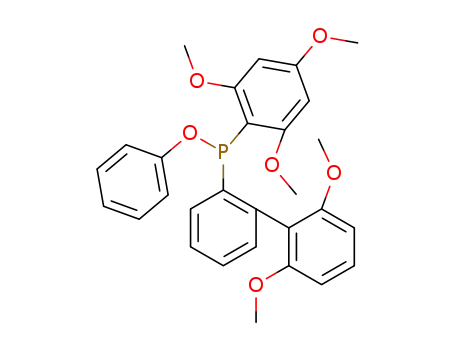
phenyl 2-(2',6'-dimethoxybiphenylyl)(2,4,6-trimethoxyphenyl)phosphinite
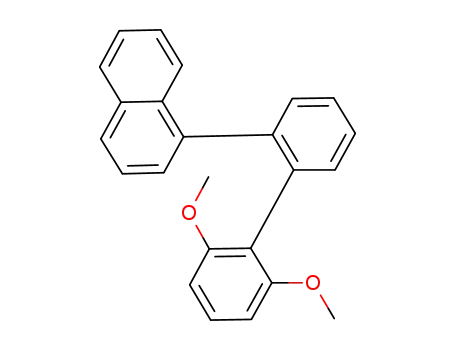
2,6-dimethoxy-2'-naphthalen-1-ylbiphenyl
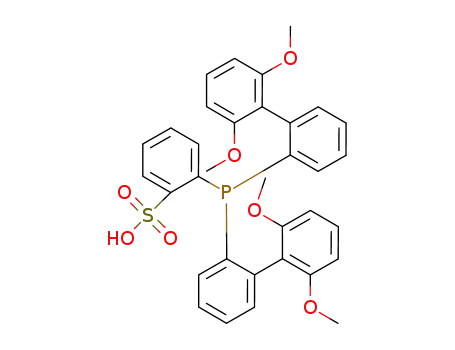
(2-(2',6'-(OMe)2-C6H3)-C6H4)2PH(2-SO3-C6H4)
CAS:1002345-50-7
Molecular Formula:C27H52B2F8P2
Molecular Weight:612.3
CAS:1606981-69-4