Your Location:Home >Products >OLED intermediates >Carbazoles >35883-22-8

Product Details
Palladium-catalysed Buchwald–Hartwig amination of ortho-substituted hindered aryl bromides or chlorides with 9H-carbazole has been investigated. In the amination of 1-bromo- or chloronaphthalene with 9H-carbazole, the combined use of Pd2(dba)s
PROBLEM TO BE SOLVED: To efficiently produce 9-(2-substituted phenyl)-9H-carbazole. SOLUTION: A 9-(2-substituted phenyl)-9H-carbazole is produced by causing a reaction to occur between a 9H-carbazole derivative and 2-substituted-1-halobenzene in the prese
A highly site-selective, one-pot, sequential C-N and C-C bond forming process was developed, affording a carbazole-based skeleton that contains biphenyl and diarylacetylene cores. The success of this process is attributed to the use of fluorinated iodoarenes as the starting material, the fluorine group of which preferentially reacts with carbazole. The subsequent coupling of the intermediate iodinated N-arylcarbazole with arylboronic acid or arylacetylene produced the desired products. The intermediate underwent a Pd-catalyzed Ullmann coupling with excess fluorinated iodoarenes in the absence of arylboronic acid or arylacetylene, resulting in Ullmann coupling products in a one-pot process.
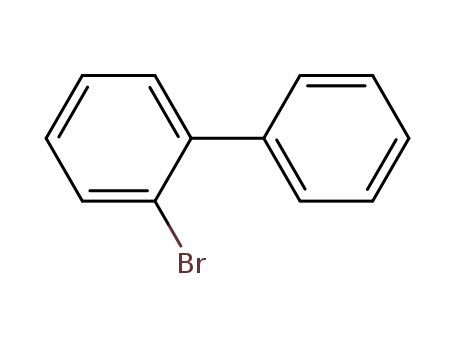
2-Bromobiphenyl

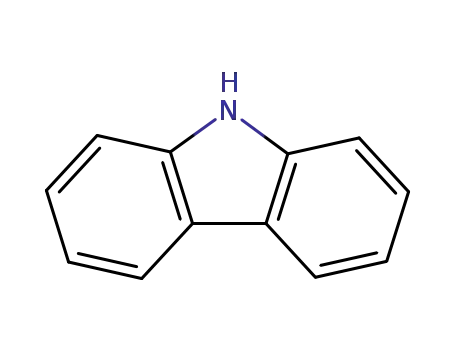
9H-carbazole

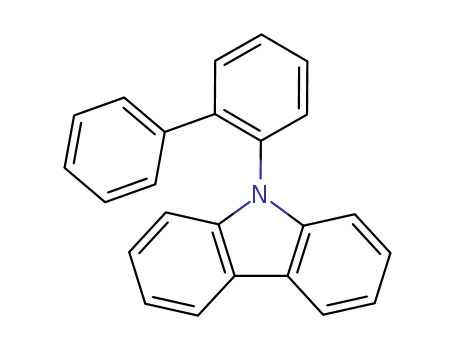
![9-([1,1'-biphenyl]-2-yl)-9H-carbazole](/upload/2023/2/d6ec5671-1421-48a8-8a5e-3839fab4972d.png)
9-([1,1'-biphenyl]-2-yl)-9H-carbazole
| Conditions | Yield |
|---|---|
|
With
tris-(dibenzylideneacetone)dipalladium(0); N,N′-bis(2,6-bis(diphenylmethyl)-4-methoxyphenyl)imidazol-2-ylidene; lithium hexamethyldisilazane;
In
5,5-dimethyl-1,3-cyclohexadiene; toluene;
at 140 ℃;
for 15h;
Reagent/catalyst;
Solvent;
Catalytic behavior;
Inert atmosphere;
Sealed tube;
|
83% |
|
With
tris-(dibenzylideneacetone)dipalladium(0); N,N′-bis(2,6-bis(diphenylmethyl)-4-methoxyphenyl)imidazol-2-ylidene; lithium hexamethyldisilazane;
In
5,5-dimethyl-1,3-cyclohexadiene; toluene;
at 140 ℃;
for 15h;
Reagent/catalyst;
Solvent;
Temperature;
Inert atmosphere;
|
83% |
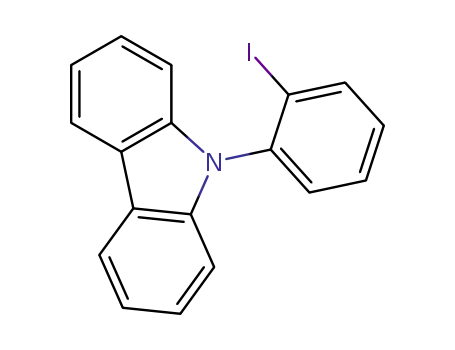
9-(2-iodophenyl)-9H-carbazole

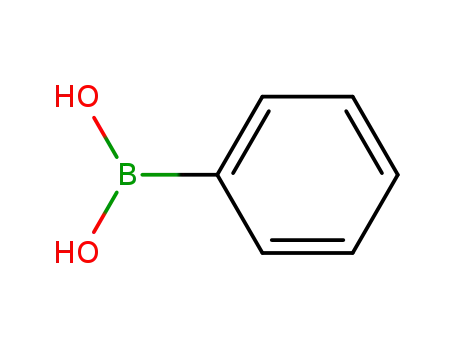
phenylboronic acid

![9-([1,1'-biphenyl]-2-yl)-9H-carbazole](/upload/2023/2/d6ec5671-1421-48a8-8a5e-3839fab4972d.png)
9-([1,1'-biphenyl]-2-yl)-9H-carbazole
| Conditions | Yield |
|---|---|
|
With
triphenylphosphine; palladium dichloride;
In
N,N-dimethyl acetamide;
at 120 ℃;
for 6h;
|
147 mg |

phenylboronic acid

9H-carbazole
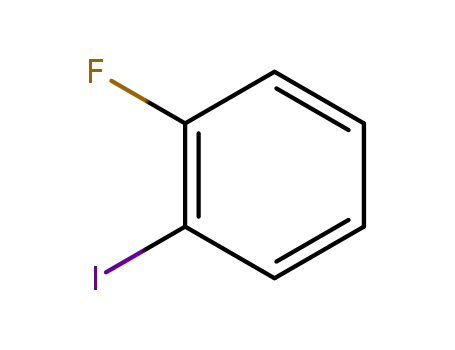
1-Fluoro-2-iodobenzene
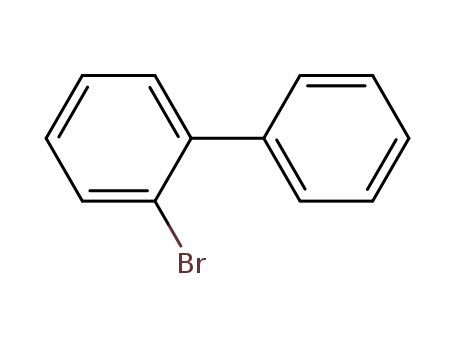
2-Bromobiphenyl
CAS:605644-46-0
CAS:2622-14-2
CAS:1257220-52-2
CAS:22034-43-1